Photo credits: ScenTree SAS
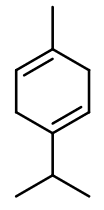
Gamma-Terpinene
Menthadiene-para ; 1-methyl-4-propan-2-ylcyclohexa-1,4-diene ; Crithmene ; 1-methyl-4-isopropyl-1,4-cyclohexadiene ; Para-mentha-1,4-diene ; 4-methyl-1-(1-methylethyl)-1,4-cyclohexadiene ; 1-methyl-4-(1-methyl ethyl)-1,4-cyclohexadiene ; Moslene ; 1-isopropyl-4-methyl-1,4-cyclohexadiene ; 4-isopropyl-1-methyl-1,4-cyclohexadiene

Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 99-85-4
-
EINECS number : 202-794-6
-
FEMA number : 3559
-
FLAVIS number : 01.020
-
JECFA number : 1340
-
Appearance : Colorless liquid
-
Density : 0,847
-
Volatility : Head
-
Price Range : €
Physico-chemical properties
-
Molecular formula : C10H16
-
Molecular Weight : 136,24 g/mol
-
Log P : 4,5
-
Fusion Point : -10°C
-
Boiling Point : 182°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 56°C
Uses
Uses in perfumery :
Gamma-Terpinene is used in fresh and zesty citrus notes, for a top note contribution. Gives a terpenic effect to a citrus note. Widely used in consumer products, detergents and other household products.
Year of discovery :
Data not available.
Natural availability :
Gamma-Terpinene is present in a large quantity (around 20%) in Ajowan EO, a seed grown in southern India, botanically close to caraway and cumin. This molecule is also found in Bergamot EO, Cumin EO, Cajuput EO, Eucalyptus delegatensis EO (Ethiopian variety) and Grapefruit EO among others. It can be extracted from all these essential oils by fractional distillation.
Isomerism :
Gamma-Terpinene is the isomer of many other terpenes, including D-Limonene, alpha-Pinene and beta-Pinene and alpha-Terpinene. The latter isomer has a smell that is quite close to gamma-Terpinene, although it is closer to Lime EO, and slightly less terpenic.
Synthesis precursor :
Gamma-Terpinene can be used for the synthesis of other terpenses and sesquiterpenes, always by Diels-Alder reaction. It can be used in other various reactions, such as a hydrolysis reaction. Its structure has a wide range of possible reactions.
Synthesis route :
Like many terpenes, the reaction to obtain Gamma-Terpinene is a Diels-Alder reaction. It involves reacting a molecule called ''diene '' with another called ''dienophile '' to cyclize the molecule. Here, Isoprene (diene) can react with 3-methylbutyne (dienophile). Therefore, gamma-Terpinene results directly from this reaction.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

