Photo credits: ScenTree SAS
Gamma-Decalactone
5-hexyloxolan-2-one ; 2-decalactone ; Decan-4-olide ; Decano-1,4-lactone ; Alpha-decanolactone ; 4-decanolide ; Decanolide-1,4 ; 4,5-dihydro-5-hexyl-2(3H)-furanone ; 4-hexyl butan-4-olide ; 4-hexyl-4-butanolide ; 4-hexyl-gamma-butyrolactone ; Gamma-hexyl-gamma-butyrolactone ; 4-hexylbutan-4-olide ; 5-hexyloxolan-2-one

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
(R) GAMMA DECALACTONE | M_0050448 |
Visit website
|
Naturel | - | - | - | - | - |
General Presentation
-
CAS N° : 706-14-9
-
EINECS number : 211-892-8
-
FEMA number : 2360
-
FLAVIS number : 10.017
-
JECFA number : 231
-
Appearance : Colorless liquid
-
Density : 0,967
-
Volatility : Heart/Base
-
Price Range : €€€
Physico-chemical properties
-
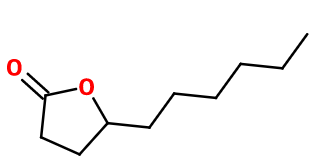
Molecular formula : C10H18O2
-
Molecular Weight : 170,25 g/mol
-
Log P : 3
-
Fusion Point : <-20°C
-
Boiling Point : 281°C
-
Detection Threshold : 11 ppb (0,0000011%)
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 150°C
Uses
Uses in perfumery :
Gamma-Decalactone adds a fruity and velvety touch to floral accords. Used in peach reconstitutions, accompanied by Aldehyde C14.
Year of discovery :
Data not available.
Natural availability :
Gamma-Decalactone is included in the fragrant principle of many fruits such as apricot or peach, and can be extracted from Osmanthus Absolute or from Carrot Leaf EO.
Isomerism :
Gamma-Decalactone has an asymmetric carbon. However, it is its racemic mixture that is used in perfumery. Delta-Decalactone is a constitutional isomer of Gamma-Decalactone, having one carbon atom less in its cycle, and one more in its ramified carbon chain. The resulting smell is very peachy for the isomer, and coconut-like for Delta-Decalactone.
Synthesis precursor :
Gamma-Decalactone is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Gamma-Decalactone is part of the family of gamma-lactones: cyclic esters whose cycle consists of five atoms. This molecule is synthesized biochemically. A beta-oxidation of ricinoleic acid (derived from castor oil) causes the formation of 4-Hydroxydecanoic acid, which can be cyclized at an acidic pH to obtain the final product: gamma-Decalactone.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

