Photo credits: ScenTree SAS
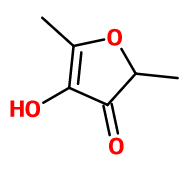
Furaneol®
Fraision® ; Furonol ; Strawberry Furanol ; 4-hydroxy-2,5-dimethylfuran-3-one ; Alletone ; Alnose ; Cadion ; Carmelan ; Dimethyl hydroxyfuranone ; 2,5-dimethyl-3-hydroxy-4-oxo-4,5-dihydrofuran ; 2,5-dimethyl-4-hydroxy-3-furanone ; Enhansol ; Flerueol ; Furanone ; Furonol ; 4-hydroxy-2,5-dimethyl-2-hydrofuran-3-one ; 4-hydroxy-2,5-dimethylfuran-3-one ; Pineapple ketone ; Strawberry furanone ; Walnut furanone

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Furaneol - 30 Gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 3658-77-3
-
EINECS number : 222-908-8
-
FEMA number : 3174
-
FLAVIS number : 13.010
-
JECFA number : 1446
-
Appearance : White solid
-
Density : 1,322
-
Volatility : Head/Heart
-
Price Range : €€€
Physico-chemical properties
-
Molecular formula : C6H8O3
-
Molecular Weight : 128,13 g/mol
-
Log P : 0,95
-
Fusion Point : 79°C
-
Boiling Point : 230°C
-
Detection Threshold : 0,03 ppb à 60 ppb (0,000006%) selon les personnes
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : >100°C (>212°F)
Uses
Uses in perfumery :
Furaneol®makes it possible to add a burnt, sweet, jammy and gourmet note to a fruity accord. Used for strawberry and pineapple accords in particular.
Year of discovery :
''Furaneol® '' tradename has been published and protected by Firmenich SA since 31/10/1973 (brand N°402723)
Natural availability :
Natural Furaneol® can be extracted from certain fruits and is formed by pyrogenation of some food (almond for example). In the majority of cases, it is synthetic Furaneol® that is used in perfumery.
Isomerism :
Furaneol® has no isomer commonly used in perfumery.
Synthesis precursor :
Furaneol® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Furaneol® can be synthesized in two main ways. Only one is exposed here: it consists firstly in an ethylation of Acetaldehyde (or ethanal), to synthesize 2,5-hexynediol. The second step is an ozonolysis of this compound in order to obtain hexane-2,5-diol-3,4-dione, which can finally be cyclized in an acid medium to give Furaneol®.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,045 % 0,014 % 0,27 % 0,25 % 0,064 % 0,064 % 0,064 % 0,021 %0,15 % Cat.5A B C DCat.6 0,064 % 0,064 % 0,064 % 0,021 %0,15 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,52 % 0,52 %0,021 % 0,49 % 0,49 % 1,8 %0,021 % 0,021 %No Restriction Cat.10A BCat.11A BCat.12 0,49 % 1,8 %0,021 % 0,021 %No Restriction
-
Contributions from other sources
4-Hydroxy-2,5-dimethyl-3(2H)-furanone has been found in natural extracts but only at trace levels.


