Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 125109-85-5
-
EINECS number : 412-050-4
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,95
-
Volatility : Heart
-
Price Range : €€€
Physico-chemical properties
-
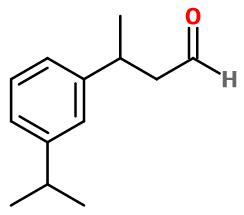
Molecular formula : C13H18O
-
Molecular Weight : 190,29 g/mol
-
Log P : 3,8
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 257°C
-
Detection Threshold : 0,07 ng/l air
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 68°C
Uses
Uses in perfumery :
Florhydral® is generally used to create lily of the valley or hyacinth notes. It is especially used for reformulating Lilial®-containing perfumes. Its notes give it a good synergy with citrus notes. Used in all kinds of perfumes.
Year of discovery :
Patent N°4,910,346 (US) published on Nov. 10, 1988 by Chalk.A for Givaudan Corp.
Natural availability :
Florhydral® does not exist on a natural state. Thus, it can't be used as extracted from a plant.
Isomerism :
Florhydral® has an asymmetric carbon, giving birth to two possible enantiomers. It is anyway a blend of these two isomers that is used in perfumery. Florhydral® also is a positional isomer of Cyclamen Aldehyde, which has a relatively close smell, although more marine.
Synthesis precursor :
Florhydral® is not used for the synthesis of another molecule of olfactive interest.
Synthesis route :
Florhydral® can be synthesized by a hydroformylation reaction of 1,3-diisopropenylbenzene, using carbon monoxide and hydrogen to form an aldehydic group, starting from an alcene group.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

