Photo credits: ScenTree SAS
Floralozone®
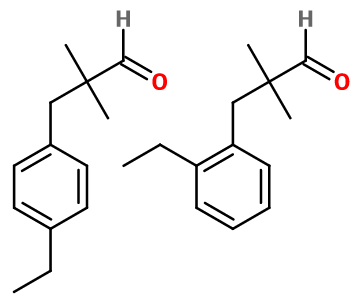
2 et 4-ethyl-2,2-dimethylbenzenepropanal ; Ortho et para-ethyl-2,2-dimethylbenzenepropanal ; Ortho et para-ethyl dimethyl cinnamaldehyde ; 3-(ortho et para-ethyl phenyl)-2,2-dimethyl propionaldehyde ; Floralozonex ; Florazon ; Florone ; Ozofloranal ; Ozone propanal

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Floralozone - 30 Gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 67634-15-5- 67634-14-4
-
EINECS number : 266-819-2, 266-818-7
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,955
-
Volatility : Heart/Base
-
Price Range : €€€
Physico-chemical properties
-
Molecular formula : C13H18O
-
Molecular Weight : 190,29 g/mol
-
Log P : 3,6
-
Fusion Point : Donnée indisponible.
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 100°C
Uses
Uses in perfumery :
Floralozone® is used to make the link between a marine note and a floral note. It also enters in lilac accords, and brings a rubbery effect if overdosed.
Year of discovery :
Data not available.
Natural availability :
Floralozone® is not found in nature, and can therefor not be extracted.
Isomerism :
Floralozone® consists of two positional isomers: ortho and para. These two isomers are formed during the synthesis of Floralozone®, and are not used separately. Floralozone® is also a positional isomer of Cyclamen Aldehyde. Both have a marine and floral note, but differ in an aniseed character for Floralozone®, and a more aldehydic note for Cyclamen Aldehyde.
Synthesis precursor :
Floralozone® is not a precursor for the synthesis of another compound of olfactive interest.
Synthesis route :
Floralozone® can be synthesized in two steps from benzene. The first step is a Friedel and Craft alkylation reaction. This reaction uses for example chloroethane, reacting with a Lewis acid such as aluminium chloride. By adding benzene under pressure, it is possible to obtain ethylbenzene. In the second step, 4-chloro-2,2-dimethylpropanal is reacted in the same way with the intermediate product formed, after reacting with the same Lewis acid. The two isomers obtained are then the ortho and para isomers of ethyl 2,2-dimethylbenzenepropanal.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

