Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
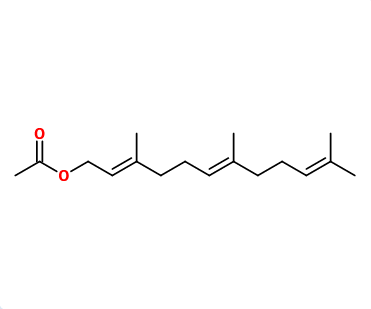
CAS N° : 29548-30-9
-
EINECS number : 249-689-1
-
FEMA number : 4213
-
FLAVIS number : 09.818
-
JECFA number : 1831
-
Appearance : Colorless liquid
-
Density : 0,91
-
Volatility : Heart/Base
-
Price Range : €€€€
Physico-chemical properties
-
Molecular formula : C17H28O3
-
Molecular Weight : 264,4 g/mol
-
Log P : 6,77
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 325°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 110°C
Uses
Uses in perfumery :
Mainly used to add volume and freshness in white flowers accords.
Year of discovery :
Data not available.
Natural availability :
Found in Ambrette seed Oil (4%)
Isomerism :
Due to the presence of two double bonds in Farnesol, there are four pairs of diastereoisomers of Farnesyl acetate : (E,E), (Z,E), (E,Z), (Z,Z). In perfumery, we use a mix of all those isomers.
Synthesis precursor :
Farnesyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
The synthesis of Farnesyl acetate is carried out by an esterification reaction between Acetic Acid and Farnesol. This reaction can be catalyzed by the presence of a small amount of a strong acid such as concentrated sulfuric acid. It can also be achieved by thanks to the use of acetic anhydride or chloroacetic acid instead of acetic acid.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

