Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Eucalyptol - 30gr | - |
Visit website
|
- | - | - | - | - | - | |
|
|
EUCALYPTOL | M_0053330 |
Visit website
|
Naturel | - | - | - | - | - |
General Presentation
-
CAS N° : 470-82-6
-
EINECS number : 207-431-5
-
FEMA number : 2465
-
FLAVIS number : 03.001
-
JECFA number : 1234
-
Appearance : Colorless liquid
-
Density : 0,925
-
Volatility : Head
-
Price Range : €€
Physico-chemical properties
-
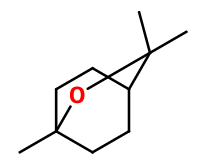
Molecular formula : C10H18O
-
Molecular Weight : 154,25 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : 1°C
-
Boiling Point : 176°C
-
Detection Threshold : 1 et 64 ppb (0,0000064%)
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 48°C
Uses
Uses in perfumery :
Eucalyptol is used in woody, aromatic and fougere perfumes, to boost a lavender and to ice flowers and fruits, bringing them a cold facet.
Year of discovery :
Data not available.
Natural availability :
Eucalyptus EO contains up to 85% Eucalyptol, therefore the molecule can be extracted with a purity of up to 99,8%. Bay Leaf EO also contains up to 70% Eucalyptol.
Isomerism :
Eucalyptol has several constitutional isomers, mainly with a floral note: Linalool, Geraniol and Nerol are part of it. Menthone® is also an isomer of Eucalyptol, and has a very minty note, but less coniferous.
Synthesis precursor :
Eucalyptol can be a precursor to the synthesis of other terpenes, by a Diels-Alder reaction for example.
Synthesis route :
Eucalyptol is a little synthesized raw material, as it can be extracted from Eucalyptus EO and other oils with a high yield and high purity.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

