Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Ebanol® - 30gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 67801-20-1
-
EINECS number : 267-140-4
-
FEMA number : 4775
-
FLAVIS number : Donnée indisponible.
-
JECFA number : 2220
-
Appearance : Colorless liquid
-
Density : 0,9
-
Volatility : Base
-
Price Range : €€
Physico-chemical properties
-
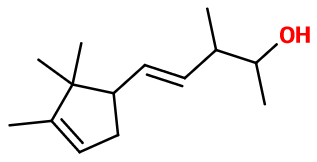
Molecular formula : C14H24O
-
Molecular Weight : 208,34 g/mol
-
Log P : 4,9
-
Fusion Point : <-50°C
-
Boiling Point : 283°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 108°C
Uses
Uses in perfumery :
Ebanol® is used to bring volume and elegance to woody accords, with a typical sandalwood and diffusive note. It brings great tenacity in perfumes.
Year of discovery :
1986
Natural availability :
Ebanol® is not found in nature. Thus, it cannot be extracted from any plant.
Isomerism :
Ebanol® is a mixture of four diastereoisomers. These isomers appear during the synthesis of this material and are due to three asymmetric carbons and one double bond that is giving birth to two isomers inside the molecule. Among these isomers, trans-(E)-Ebanol® and cis-(Z)-Ebanol® are important. Then, a mixture of these isomers is used in perfumery.
Synthesis precursor :
Ebanol® is not used for the synthesis of any other material used in perfumery.
Synthesis route :
Ebanol® can be synthesized in three steps. The first one is a condensation of Campholenaldehyde with 2-butanone. The obtained intermediary product is isomerized by adding potassium tert-butylate, diluted in dimethyl formamide. The last step is a reduction of the product with sodium tetrahydruroborate, leading to four stereoisomers (see ''Isomerism '' paragraph).
Regulations & IFRA
Allergens :
This ingredient is classified as an allergen under European Regulation 2023/1545, dated August 26, 2023.
Its presence must therefore be declared on product labels when it exceeds 0.001% in leave-on products and 0.01% in rinse-off products.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

