Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Dihydromyrcenol - 30 Gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 18479-58-8
-
EINECS number : 242-362-4
-
FEMA number : Donnée indisponible.
-
FLAVIS number : 02.144
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,784
-
Volatility : Heart
-
Price Range : €€
Physico-chemical properties
-
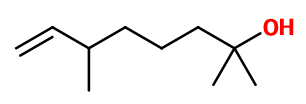
Molecular formula : C10H20O
-
Molecular Weight : 156,27 g/mol
-
Log P : 3,25
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 196°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 77°C
Uses
Uses in perfumery :
Dihydromyrcenol is used in zesty and fougere accords, and to give a fresh nuance to fruity notes.
Year of discovery :
1956
Natural availability :
Dihydromyrcenol is very poorly present in nature. It is therefore in most cases the synthetic version that is used in perfumery.
Isomerism :
Dihydromyrcenol has an asymmetric carbon. However, it is the racemic mixture of the two enantiomers that is used in perfumery. Citronellol and Rosalava are constitutional isomers of Dihydromyrcenol. Both also have a rosy smell, more lemony for Citronellol and more intense for Rosalva®.
Synthesis precursor :
Dihydromyrcenol is an intermediary in the synthesis of Tetrahydromyrcenol and a reagent in the synthesis of Dihydromyrcenyl acetate and other esters of this alcohol.
Synthesis route :
The synthesis of Dihydromyrcenol starts from cis-pinane, which is pyrolyzed to give an intermediate product called Citronellene. To obtain Dihydromyrcenol, the process can be achieved in three different ways : the first one is to put hydrochloric acid in contact with Citronellene, followed by a hydrolysis. The second is the addition of formic acid to saponify the resulting Dihydromyrcenyl Formate. The last one is a direct acid hydrolysis in the presence of sulfuric acid.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

