Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Boisambrene forte - 30 Gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 58567-11-6
-
EINECS number : 261-332-1
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,931
-
Volatility : Base
-
Price Range : €€€
Physico-chemical properties
-
Molecular formula : C15H30O2
-
Molecular Weight : 242,4 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 94°C (à 0,1 Pa)
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 150°C
Uses
Uses in perfumery :
Boisambrene Forte® is used in woody and ambery notes to bring strength and its characteristic note. This compound can also be used in alkaline bases such as soaps or detergents, thanks to its stability.
Year of discovery :
Discovered in 1974.
Natural availability :
Boisambrene Forte® is not available in its natural state.
Isomerism :
Boisambrene Forte® does not have any isomer used in perfumery.
Synthesis precursor :
Boisambrene Forte® is not a precursor to the synthesis of antoher compound of olfactory interest.
Synthesis route :
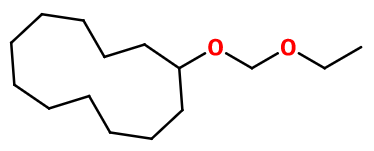
Boisambrene Forte® can be obtained by reaction between cyclododecanol and a gazeous complex of pure paraformaldehyde and hydrochloric acid. The intermediary product is called chloromethoxy cyclododecane. This byproduct can undergo an ethylation reaction with sodium ethanolate, prepared with pure sodium in ethanol.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,27 % 0,080 % 1,6 % 1,5 % 0,38 % 0,38 % 0,38 % 0,13 %0,49 % Cat.5A B C DCat.6 0,38 % 0,38 % 0,38 % 0,13 %0,49 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 3,1 % 3,1 %0,13 % 2,9 % 11 % 11 %0,13 % 0,13 %No restriction Cat.10A BCat.11A BCat.12 11 % 11 %0,13 % 0,13 %No restriction


