Photo credits: ScenTree SAS
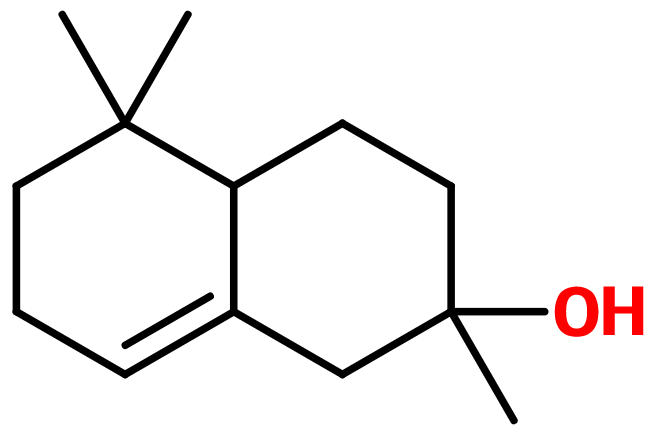
Ambrinol
Ambrinol S ; Amber naphtalenol ; Ambrinol-alpha ; Alpha-ambrinol ; Ambrinol 95 ; 2-hydroxy-2,5,5-trimethyl-1,2,3,4,4a,5,6,7-octahydronaphtalene ; 1,2,3,4,4a,5,6,7-octahydro-2,5,5-trimethyl-2-naphtalenol ; 2,5,5-trimethyl-octahydro-2-naphtalenol

Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 41199-19-3
-
EINECS number : 255-256-8
-
FEMA number : Donnée indisponible.
-
FLAVIS number : 02.197
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,94
-
Volatility : Base
-
Price Range : €€€€
Physico-chemical properties
-
Molecular formula : C13H22O
-
Molecular Weight : 194,32 g/mol
-
Log P : 4,2
-
Fusion Point : Donnée indisponible.
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : >100°C (>212°F)
Uses
Uses in perfumery :
Ambrinol is used to reproduce the natural Ambergris smell, bringing an animalic, sensual and ambery note, with a fixative effect.
Year of discovery :
Data not available.
Natural availability :
Ambrinol is one of the components of Ambergris, previously used for its sensual and animalic notes, and extracted as a tincture in alcohol. Then, only synthetic Ambrinol is used for perfumery.
Isomerism :
Ambrinol frequently used in perfumes is Alpha-Ambrinol. Beta-Ambrinol also exists, but is much less used. On the other hand, Ambrinol has one asymmetric carbon, giving birth to two possible enantiomers for this molecule. It is anyway a mixture of these isomers that is used in perfumery. Eventually, Ambrinol also is a constitutional isomer of DihydroBeta-ionone, also prepared from Beta-Ionone.
Synthesis precursor :
Ambrinol is not a precursor for the synthesis of another material used in perfumery.
Synthesis route :
Ambrinol can be synthesized thanks to Beta-Ionone. This molecule can undergo thermolysis (chemical decomposition under the effect of heat), bringing dehydroambrinol. Hydrogenation in methanol, in the presence of Raney nickel catalysor leads to Ambrinol.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

