Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 929625-08-1- 1001252-30-7
-
EINECS number : 482-030-8
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,98
-
Volatility : Base
-
Price Range : €€€€
Physico-chemical properties
-
Molecular formula : C18H30O
-
Molecular Weight : 262,48 g/mol
-
Log P : 6,3
-
Fusion Point : Donnée indisponible.
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : >93°C
Uses
Uses in perfumery :
Ambermax® is used most of the time in perfumes for power and stability reasons. It brings an strong ambery identity in any kind of perfumes, especially in fabric care products, combined with other ambery molecules as Ambroxan®, Kephalis®and Amberketal®.
Year of discovery :
2005
Natural availability :
Ambermax® is not found in nature.
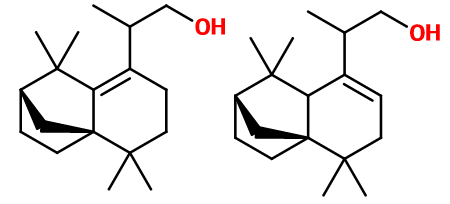
Isomerism :
Ambermax® used in perfumery is a mixture of two positional isomers, depending on the position of the double bond found in this molecule. These two isomers are formed during the synthesis of Ambermax®. They are not separated for cost reasons. Anyway, the olfactive contribution of one isomer, compared to the other, would not be so valuable.
Synthesis precursor :
Ambermax® is not a precursor for the synthesis of another material used in perfumery.
Synthesis route :
Data not available.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

