Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
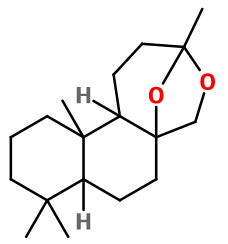
General Presentation
-
CAS N° : 57345-19-4
-
EINECS number : 260-686-4
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Pale yellow liquid
-
Density : 0,87 (dans le MIP)
-
Volatility : Base
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C18H30O2
-
Molecular Weight : 278,43 g/mol
-
Log P : 4,59
-
Fusion Point : Donnée indisponible.
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 102°C
Uses
Uses in perfumery :
According to its olfactive weakness, this woody and ambery molecule is less used than some others. Amberketal® can be used in woody-ambergris notes and especially in functionnal products thanks to its stability.
Year of discovery :
Discovered in 1953.
Natural availability :
Amberketal® is not available in its natural state.
Isomerism :
Amberketal has got five asymmetric carbons, forming a few possible isomers. Nevertheless, only a mix of these isomers is used in perfumery.
Synthesis precursor :
Amberketal® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Amberketal® is synthesized from manool, a natural component extracted from ''pink pine '' tree Halocarpus biformis, growing in New-Zealand. One of Manool's double bond is epoxidized, subsequent oxydative degradation of the allyl alcohol function into a ketone, and an intramolecular acetalization, giving birth to the final ketal function.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

