Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
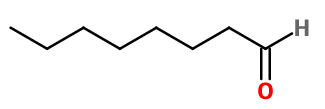
OCTANAL | M_0053353 |
Visit website
|
Naturel | - | - | - | - | - |
General Presentation
-
CAS N° : 124-13-0
-
EINECS number : 204-683-8
-
FEMA number : 2797
-
FLAVIS number : 05.009
-
JECFA number : 98
-
Appearance : Colorless liquid
-
Density : 0,822
-
Volatility : Head
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C8H16O
-
Molecular Weight : 128,21 g/mol
-
Log P : 2,9
-
Fusion Point : 14°C
-
Boiling Point : 170°C
-
Detection Threshold : 0,6276 ng/l air
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 52°C
Uses
Uses in perfumery :
Aldehyde C-8 is used in floral-aldehydic notes, citrus reproductions and top notes in general for the aldehydes metallic characteristic note. Top notes booster.
Year of discovery :
Data not available.
Natural availability :
Aldehyde C-8 is naturally present in many citrus extracts such as Bergamot EO or Sweet Orange EO, from which it can be extracted.
Isomerism :
1,3 Octenol and Methyl Hexyl Ketone are constitutional isomers of Aldehyde C-8. However, their smell is very different, as they are more earthy and reminiscent of mushroom.
Synthesis precursor :
Aldehyde C-8 forms a Schiff base by reaction with Methyl Anthranilate or Indole for example. It can also be used for condensation with another aldehyde or ketone to form the desired alcene.
Synthesis route :
Aldehyde C-8 is an aliphatic aldehyde. Like the other aldehydes, it can be synthesized by reaction of octyl halides (chloride, for example) and dimethyl sulfoxide (DMSO), followed by an alkaline treatment with sodium bicarbonate. Other synthetic routes exist such as a reduction of Rosenmund from octylic acid, using an acid chloride.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

