Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Aldéhyde C11 Undécylénique - 30gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 112-45-8
-
EINECS number : 203-973-1
-
FEMA number : 3095
-
FLAVIS number : 05.035
-
JECFA number : 330
-
Appearance : Colorless liquid
-
Density : 0,844
-
Volatility : Head
-
Price Range : €€
Physico-chemical properties
-
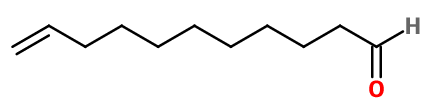
Molecular formula : C11H20O
-
Molecular Weight : 168,28 g/mol
-
Log P : 5,1
-
Fusion Point : 7°C
-
Boiling Point : 235°C
-
Detection Threshold : 2,8541 ng/l air
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 96°C
Uses
Uses in perfumery :
Aldehyde C-11 Undecylenic provides an aldehydic facet in green, chypre, rose, floral-violet, tuberose notes. Used in detergents for its stability.
Year of discovery :
Data not available.
Natural availability :
Aldehyde C-11 Undecylenic has been identified as present in trace amounts in Coriander Leaf EO, but is not extracted in its natural state.
Isomerism :
Ethyl Linalool is a constitutional isomer of Aldehyde C-11 Undecylenic. Its smell is however much more floral-fresh and less zesty.
Synthesis precursor :
Aldehyde C-11 undecylenic forms a Schiff base by reaction with Methyl Anthranilate or Indole, for example. It can also be used for condensation with another aldehyde or ketone to form the desired alcene.
Synthesis route :
Like the other aliphatic aldehydes, Aldehyde C-11 undecylenic, can be synthesized by a reaction of undecylenyl halides (chloride, for example) with dimethyl sulfoxide (DMSO), followed by an alkaline treatment with sodium bicarbonate. Other synthetic routes exist, such as a reduction of Rosenmund from undecylenic acid, using an acid chloride.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

