Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 25773-40-4
-
EINECS number : 247-256-1
-
FEMA number : 3358
-
FLAVIS number : 14.057
-
JECFA number : Donnée indisponible.
-
Appearance : Pale yellow to yellow liquid
-
Density : 1
-
Volatility : Head
-
Price Range : €€€€
Physico-chemical properties
-
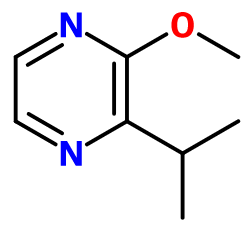
Molecular formula : C8H12N2O
-
Molecular Weight : 152,2 g/mol
-
Log P : 2,37
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 123°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 67°C
Uses
Uses in perfumery :
2-methoxy-3-isopropyl Pyrazine is used in green tomato leaves notes, and can give a galbanum nuance to modern chypre perfumes. It can also be used as a replacer of Galbanum EO to bring a raw vegetables effect.
Year of discovery :
Data not available.
Natural availability :
2-methoxy-3-isopropyl Pyrazine can be found in common plants as aspargus, peas and cucumber, but is not extracted from these plants for its use in perfumes.
Isomerism :
During the synthesis of 2-methoxy-3-isopropyl Pyrazine, two other compounds are formed : 2-methoxy-5-isopropyl Pyrazine and 2-methoxy-6-isopropyl Pyrazine. These two molecules have a similar smell as their isomer.
Synthesis precursor :
2-methoxy-3-isopropyl Pyrazine is not a precursor for the synthesis of another olfactive compound.
Synthesis route :
2-methoxy-3-isopropyl Pyrazine is part of a family of molecules which scent is close to peanuts as well as cooked and roasted fruits. Pyrazines are often obtained with a Gutknecht or Gastaldi condensation reaction, in both cases to condense two amines on two ketones, under the effect of an acid and oxidation. Here, one of the reagents contains both methoxy and isopropyl groups of the final molecule, while the other does not have any branches. Another synthesis way, in two steps, consists in reacting Glycoxal with Norvaline amide hydrochloride, to form 2-hydroxy-3-propyl Pyrazine. The second step makes this intermediairy react with diazomethane, to provoke its ethylation.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

