Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Cis-3-Hexenol - 30 Gr | - |
Visit website
|
- | - | - | - | - | - | |
|
|
CIS-3-HEXENOL | M_0050638 |
Visit website
|
Naturel | - | - | - | - | - | |
|
|
CIS-3-HEXEN-1-OL | 441H075000 |
Visit website
|
Synthetic Aroma Chemicals |

|
- | - | - | Japan | - |
General Presentation
-
CAS N° : 928-96-1
-
EINECS number : 213-192-8
-
FEMA number : 2563
-
FLAVIS number : 02.056
-
JECFA number : 315
-
Appearance : Colorless liquid
-
Density : 0,848
-
Volatility : Head
-
Price Range : €
Physico-chemical properties
-
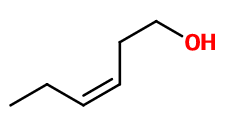
Molecular formula : C6H12O
-
Molecular Weight : 100,16 g/mol
-
Log P : 1
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 157°C
-
Detection Threshold : De l'ordre de 70 ppb (0,000007%)
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 44°C
Uses
Uses in perfumery :
Cis-3-Hexenol gives a head to floral and fruity notes. Used for the reconstitution of cut grass notes, usable in all types of perfumes.
Year of discovery :
1938
Natural availability :
Cis-3-Hexenol is present in grass, leaves, fruits (not ripe), apple and Lemongrass EO among others. It can be extracted from many plants. For example, acacia or white mulberry leaves contain more than 50% of this compound.
Isomerism :
Cis-3-Hexenol has a double bond that gives rise to two diastereoisomers of this molecule. Thus, there is trans-3-Hexenol, which has a more floral and petal smell. Trans-2-Hexenol is a positional isomer of cis-3-Hexenol because its double bond is simply delocalized. Both have a very green smell, although trans-2-Hexenol is fruty.
Synthesis precursor :
Cis-3-Hexenol is a precursor for the synthesis of several compounds of olfactory interest. For example, several of its esters such as cis-3-Hexenyl acetate or cis-3-Hexenyl Benzoate are obtained by esterification with the suitable acid, in the presence of an acid catalyst.
Synthesis route :
A synthesis of cis-3-Hexenol consists of three steps. The first one is an ethylation reaction of sodium ethylide into butyne. Then, a reaction with ethylene oxide gives 3-Hexyn-1-ol. This intermediate product is subjected to a hydrogenation reaction, catalysed by palladium. Nowadays, several biochemical synthesis processes gradually replace the organic synthesis processes.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

