Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
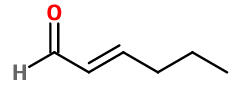
Trans-2-Hexènal - 30gr | - |
Visit website
|
- | - | - | - | - | - | |
|
|
TRANS-2-HEXENAL | 2311188610 |
Visit website
|
Synthetic Aroma Chemicals |

|
- | - | - | Romania | - |
General Presentation
-
CAS N° : 6728-26-3
-
EINECS number : 229-778-1
-
FEMA number : 2560
-
FLAVIS number : 05.073
-
JECFA number : 1353
-
Appearance : Colorless liquid
-
Density : 0,846
-
Volatility : Head
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C6H10O
-
Molecular Weight : 98,14 g/mol
-
Log P : 1,58
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 146°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 43°C
Uses
Uses in perfumery :
Trans-2-Hexenal is most often used in green notes and apple reconstitutions, to bring crunch to the fruit and this characteristic green note.
Year of discovery :
Data not available.
Natural availability :
Trans-2-Hexenal is present in several plant extracts, including the leaves of some trees and shrubs. These extracts include Geranium EO, Verbena EO or Petitgrain Grapefruit EO. It is also present in the fragrant principle of many fruits, always in small quantities. It is its synthetic version that remains the most widely used in perfumery.
Isomerism :
Trans-2-Hexenal is a diastereoisomer of cis-2-Hexenal, much less used in perfumery, but also with a very green and fruity note.
Synthesis precursor :
Trans-2-Hexenal, like all aldehydes, can be used to synthesize Schiff bases, by reaction with Methyl Anthranilate or Indole for example. These reactions often lead to the formation of a very powerful and coloured product.
Synthesis route :
The synthesis of trans-2-Hexenal can be achieved by reaction between two molar equivalents of butanal and one equivalent of ethyl vinyl ether, in the catalytic presence of boron trifluoride. This first synthesis step is followed by an acid hydrolysis of the obtained product, in the presence of concentrated sulphuric acid for example. Biosynthetic ways of obtaining this molecule are being developed, improving its yield and reducing the rejections in the environment.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,0014 % 0,00041 % 0,0083 % 0,0077 % 0,0020 % 0,0020 % 0,0020 % 0,00067 %0,0045 % Cat.5A B C DCat.6 0,0020 % 0,0020 % 0,0020 % 0,00067 %0,0045 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,016 % 0,016 %0,00067 % 0,015 % 0,054 % 0,054 %0,00067 % 0,00067 %No restriction Cat.10A BCat.11A BCat.12 0,054 % 0,054 %0,00067 % 0,00067 %No restriction
Annexe I :
Some regulated synthetic ingredients are found in nature and in certain proportions in natural ingredients. This presence in nature has to be taken into account when calculating limits of use recommended by the IFRA. In case you do not know these concentrations, you can use the ones estimated by the IFRA. Here they are :
| List of regulated compounds contained in this ingredient | |||
|---|---|---|---|
| Ingredient Name | Botanical Name | CAS N° | Estimated Concentration |
| Hyssop oil | Hyssopus officinalis L. | 8006-83-5 | 0,2 |
| Spearmint oil | Mentha spicata L. | 8008-79-5 | 0,02 |
| Erospicata oil, Mentha spicata 'Erospicata' | Mentha spicata Erospicata | 917-841-2 | 0,07 |
| Spearmint oil terpenes | Mentha spicata L. syn Mentha viridis L. var. crispa Benth. | 0,25 | |


