Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
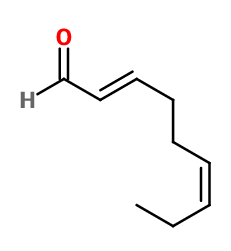
2,6-NONADIENAL WITH ATP | 441N030000 |
Visit website
|
Synthetic Aroma Chemicals |

|
- | - | - | UK | - |
General Presentation
-
CAS N° : 557-48-2
-
EINECS number : 209-178-6
-
FEMA number : 3377
-
FLAVIS number : 05.058
-
JECFA number : 1186
-
Appearance : Colorless liquid
-
Density : 0,85 - 0,87 @20°C
-
Volatility : Head
-
Price Range : €€€€€
Physico-chemical properties
-
Molecular formula : C9H14O
-
Molecular Weight : 138,21 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 95°C (à 18 mmHg)
-
Detection Threshold : De l'ordre du ppb, 10 millionièmes de pourcent
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 83°C
Uses
Uses in perfumery :
Trans-2-cis-6-Nonadienal is used in marine, cucumber and melon notes, to bring, in small quantities, a vegetal bouquet effect.
Year of discovery :
Data not available.
Natural availability :
Trans-2-cis-6-Nonadienal was found to be one of the major compounds responsible for the smell of Violet Leaf Absolute. It is the most used plant to isolate this molecule.
Isomerism :
The diastereoisomers of the molecule all have a relatively similar smell reminiscent of cucumber and violet leaf. Nevertheless, trans-2-cis-6-Nonadienal is the most commonly used isomer. Triplal® is a constitutional isomer of trans-2-cis-6-Nonadienal, but has a very green-cut grass smell, which is very different.
Synthesis precursor :
Trans-2-cis-6-Nonadienal forms Schiff bases in the presence of Methyl Anthralinate or Indole.
Synthesis route :
Trans-2-cis-6-Nonadienal is synthesized by a condensation of malonic acid with cis-4-Heptenal to give trans-2-cis-6-nonadienoic acid. Then, an esterification reaction with methanol in an acidic medium allows to obtain the corresponding ester. A reduction of this ester with lithium tetrahydruroaluminate allows to synthesize the corresponding alcohol. Finally, an oxidation with potassium permanganate allows to obtain the desired final aldehyde.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

