Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Acetate de Vetiveryle - 30 Gr | - | - | - | - | - | - | more | - | |
|
|
Vetiveryl Acetate | VT-005 | Natural | 100 | Chrysopogon zizanioides | Vetiver Oil | Indonesia | more | 50 Kgs | |
|
|
Vetiveryl Acetate | 4410000159 | Naturel | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 84082-84-8
-
EINECS number : 204-225-7
-
FEMA number : 4218
-
Density : 0,99
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Base
-
Price Range : €€€
-
Appearance : Amber liquid
-
FLAVIS number : 09.821
-
JECFA number : 1867
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 286°C
-
Detection Threshold : Donnée indisponible.
-
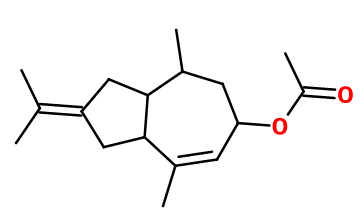
Molecular formula : C17H26O2
-
Log P : 6,16
-
Molecular Weight : 262,39 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 90°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Derivating from Vetiver Haiti EO, Vetiveryl acetate keeps a grapefruit note, compared with Vetiver Java EO, much more smoky.
Stability :
acetates may form acetic acid through time
Uses in perfumery :
Vetiveryl acetate is used in woody, ambery and chypre perfumes for its long-lasting tenacity. Goes well with floral and spicy notes. More delicate and smoky than vetiver but less faceted and woody. Both are often associated in perfumes.
Year of discovery :
Data not available.
Isomerism :
Vetiverol is not a pure compound. It is a mixture of alcohols in which the major compound is Khusimol. Therefore, Vetiveryl acetate is a mixture of esters whose major compound is Khusimyl acetate. Vetyvenal® is a constitutional isomer of Vetiveryl acetate. However, all can be used to reconstitute Vetiver EO.
Synthesis precursor :
Vetiveryl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Vetiveryl acetate is an hemi-synthetic product, derivated from Vetiver Haiti EO, and particularly from the couple of molecules called Vetiverol.
Synthesis route :
Vetiveryl acetate is prepared from the mixture of sesquiterpene alcohols obtained from Vetiver Haiti EO, Vetiverol, by an esterification reaction using acetic acid or acetic anhydride, in the presence of a catalyst like sulfuric acid. Thus, its synthesis requires a first step to isolate Vetiverol from the vetiver root, which makes it an expensive raw material.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
-
Restriction type : RESTRICTION
-
Cause of restriction : DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
-
Amendment : 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,05 % 0,05 % 0,05 % 0,9 % 0,1 % 0,1 % 0,1 % 0,033 % 0,098 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,1 % 0,1 % 0,033 % 0,2 % 0,2 % 3,8 % 0,033 % 0,033 % No Restriction


