Triplal®
Cyclal C® ; Vertocitral® ; Ligustral® ; 2,4-dimethylcyclohex-3-ene-1-carbaldehyde ; Acroval ; Citrulan ; Cyclogreenal ; 2,4-dimethyl-3-cyclohexene-1-carbaldehyde ; 4-formyl-1,3-dimethylcyclohex-1-ene ; Hivertal ; 2,4-ivy carbaldehyde ; Lantral ; Tricyclal ; Trigustral ; Trivertal ; Zestover
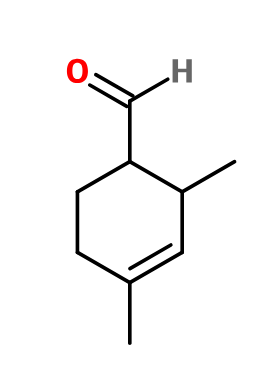
Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Triplal - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 68039-49-6
-
EINECS number : 268-264-1
-
FEMA number : 4505
-
Density : 0,937
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Head/Heart
-
Price Range : €
-
Appearance : Colorless liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C9H14O
-
Log P : 2,34
-
Molecular Weight : 138,21 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 70°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
In comparision to other cut grass notes, Triplal® has a metallic note that distinguishes it from cis-3-Hexenol for example.
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell.
Very unstable in acid cleaners, antiperspirants and very alkaline products.
Uses in perfumery :
Triplal® is used in jasmine, colognes and fougere notes, to bring a different green and fruity note and freshness. Brings a spring facet to floral notes.
Year of discovery :
1920
Isomerism :
Triplal® used in perfumery is a mixture of two enantiomers with very similar smells. This is why they are not separated by fractional distillation. Trans-2-cis-6-Nonadienal is a constitutional isomer of Triplal® but has a very different cucumber smell.
Synthesis precursor :
Triplal® forms a Schiff base with Methyl Anthranilate, with a still very green smell but fruity and more artificial.
Natural availability :
Triplal® is not available in its natural state.
Synthesis route :
Triplal® is synthesized by a Diels-Alder reaction between 2-methyl-1,3-pentadiene and acrolein. A mixture of two enantiomers is obtained.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
-
Restriction type : RESTRICTION
-
Cause of restriction : DERMAL SENSITIZATION
-
Amendment : 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,45 % 0,14 % 2,7 % 2,5 % 0,64 % 0,64 % 0,64 % 0,64 % 1,5 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 5,2 % 5,2 % 0,27 % 4,9 % 18 % 18 % 9,8 % 9,8 % No Restriction
Comments :
The above limits apply to Dimethylcyclohexen-3-ene-1-carbaldehyde (mixed isomers) used individually or in combination. The sum of concentrations of Dimethylcyclohexen-3-ene-1-carbaldehyde isomers should not exceed the maximum concentration levels established by this Standard. This ingredient is part of the Schiff base (Triplal-methyl anthranilate (or Vertosine, Ligantraal, Agrumea) - N°CAS : 68738-99-8) and induces the application of IFRA regulations for 50,9% of the Schiff base usage. Please also refer to the IFRA Annex II for more information

