Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Triéthyl Citrate - 30gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 77-93-0
-
EINECS number : 201-070-7
-
FEMA number : 3083
-
Density : 1,14
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : NON TROUVE_N/A
-
Price Range : €
-
Appearance : Colorless liquid
-
FLAVIS number : 09.512
-
JECFA number : 629
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 294°C
-
Detection Threshold : Donnée indisponible.
-
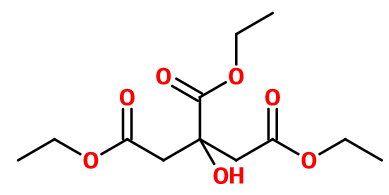
Molecular formula : C12H20O7
-
Log P : 1,17
-
Molecular Weight : 276,28 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 155°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Stability :
Data not available.
Uses in perfumery :
Triethylcitrate is a solvent commonly found in perfume concentrates, as it is mainly used to dilute raw materials that are too viscous or pasty to be used as they are.
Year of discovery :
Data not available.
Isomerism :
Triethyl Citrate does not have any isomer used in perfumery.
Synthesis precursor :
Triethyl Citrate is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Triethyl Citrate is naturally present in cabbages and some white wines. Natural Triethyl Citrate is synthesized by hemi-synthesis using natural citric acid (lemon extract, for example) and ethanol.
Synthesis route :
Triethyl Citrate is a triester of citric acid. It is therefore obtained by an extensive esterification of citric acid, by reaction with an excess of ethanol in the presence of an acid catalyst such as concentrated sulfuric acid. The end of the reaction can be evaluated by an acid-base titration: when the acid concentration is stable, the reaction is at equilibrium.
Regulations & IFRA
This ingredient is not restricted

