Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Acetate de Styrallyle - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 93-92-5
-
EINECS number : 202-288-5
-
FEMA number : 2684
-
Density : 1,025
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Head
-
Price Range : €
-
Appearance : Colorless liquid
-
FLAVIS number : 09.178
-
JECFA number : 801
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 212°C
-
Detection Threshold : Donnée indisponible.
-
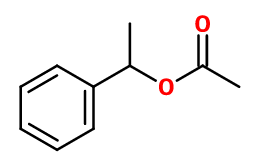
Molecular formula : C10H12O2
-
Log P : 2,5
-
Molecular Weight : 164,2 g/mol
-
Fusion Point : -60°C
-
Flash Point : 95°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Styrallyl acetate is reminiscent of rhubarb for most people. It brings a more zesty note, closing it to a molecule like Rhubafuran®, more than a more sulfuric material as Rhubofix®.
Styrallyl acetate is a major key ingredient in modern perfumery, bringing a fruity, green and pleasant effect from the top, leading to an explosive and attractive head note.
Stability :
acetates may form acetic acid through time. Aromatic molecules are said to be chromophorous. This means that they may color under the effect of light.
Uses in perfumery :
Styrallyl acetate is used in rhubarb, gardenia and tuberose notes. Gives an attack to rosy notes. Widely used in both men and women perfumery for a modern look and to dry out top notes.
Year of discovery :
1911
Isomerism :
Styrallyl acetate has an asymmetric carbon. Its (R) enantiomer has a very floral-jasmine smell, close to gardenia and fruity. The (S) enantiomer is closer to strawberry and has a green and fresh note. In perfumery, the racemic mixture of these two enantiomers is the only one that is used. Phenyl Ethyl acetate and Benzyl Propionate are constitution isomers of Styrallyl acetate. Nevertheless, Phenyl Ethyl acetate is more rosy and honeyed, and Benzyl Propionate is more floral, reminiscent of jasmine and pear.
Synthesis precursor :
Styrallyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Styrallyl acetate is available as nature identical
Synthesis route :
Styrallyl acetate results from an esterification reaction between 1-phenylethanol and acetic acid or acetic anhydride in the presence of an acid catalyst.
Regulations & IFRA
This ingredient is not restricted

