Silvial®
Alpha-methyl-4-(2-methylpropyl) benzenepropanal ; Para-isobutyl-alpha-methyl hydrocinnamaldehyde ; Aldéhyde para-isobutyl-alpha-methyl hydrocinnamique ; 3-(4-isobutylphenyl)-2-methylpropanal ; 3-(para-cumenyl)-2-methyl propionaldehyde ; Cyclamen homoaldehyde ; Homoaldehyde cyclamen ; 2-methyl-3-(4-(2-methyl propyl)phenyl) propanal ; Rhodial ; Suzaral
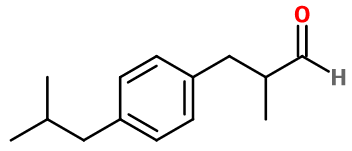
Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Silvial® - 30gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 6658-48-6
-
EINECS number : 229-695-0
-
FEMA number : Donnée indisponible.
-
Density : 0,94
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart
-
Price Range : €€
-
Appearance : Colorless liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 245°C
-
Detection Threshold : 0,7 ng/l air
-
Molecular formula : C14H20O
-
Log P : 4,7
-
Molecular Weight : 204,31 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 120°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Sylvial® has a structure close to Lilial®, Cyclamen Aldehyde®, Bourgeonal™ and Florhydral®. Each of these molecules have a light flower smell, with a more marine nuance for Cyclamen Aldehyde®, greener for Bourgeonal™ and fruitier for Florhydral® for example.
Stability :
Very unstable in stong acidic (detergents) and alkaline (liquid bleach) bases. Stable in other types of bases.
Uses in perfumery :
Silvial® is usually used to replace regulated aldehydes as Lilial® or Lyral® in lily of the valley accords for example. It brings a powerful and fresh note, with a floral identity.
Year of discovery :
Patent N°1.430.164 (FR) published on Nov.13, 1964 by Rhône-Poulenc company
Isomerism :
Silvial® has one asymmetric carbon. Nevertheless, a mixture of these two molecules is used in perfumes. Also, ortho and meta isomers of Silvial® are not used in perfumery. Eventually, Lilial® is a positional isomer of Silvial®. Its smell is fresher and fruitier, and less aqueous.
Synthesis precursor :
Reacting with Methyl Anthranilate, Silvial® can help synthesizing its Schiff base, although it is very poorly used.
Natural availability :
Sylvial® is not found in nature. Thus, it is not extracted from any plant.
Synthesis route :
As it has a structure close to Lilial® and Cyclamen Aldehyde®, the synthesis of Silvial® can be made in two different ways. The first one consists in a condensation of two aldehydes : 4-isobutylbenzaldehyde and Acetaldehyde, using an excess of the first reagent, and adding the second one slowly, to prevent autocondensation of Acetaldehyde. Then, a catalytic hydrogenation of the obtained product leads to Silvial®. The second synthetic route is a Friedel and Craft reaction involving isobutylbenzene and Methacrolein diacetate, followed by subsequent acidic hydrolysis of the intermediary product.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
-
Restriction type : RESTRICTION
-
Cause of restriction : DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
-
Amendment : 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,08 % 0,053 % 0,8 % 0,99 % 0,25 % 0,25 % 0,25 % 0,083 % 0,08 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,72 % 0,72 % 0,083 % 1,9 % 1,9 % 5,4 % 0,083 % 0,083 % No Restriction

