Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Sandalore - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 65113-99-7
-
EINECS number : 265-453-0
-
FEMA number : Donnée indisponible.
-
Density : 0,898
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Base
-
Price Range : €€
-
Appearance : Colorless viscous liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 275°C
-
Detection Threshold : Donnée indisponible.
-
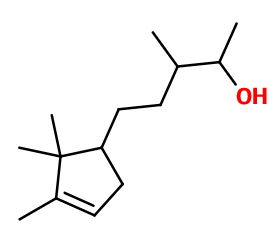
Molecular formula : C14H26O
-
Log P : 4,7
-
Molecular Weight : 210,36 g/mol
-
Fusion Point : <-50°C
-
Flash Point : 95°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Sandalore® is less milky than Sandela® and less potent than Bacdanol®.
Stability :
Unstable in acid cleaners and in very alkaline products.
Uses in perfumery :
Sandalore® is used in sandalwood reproductions, woody notes for bringing volume and trail. Used for a milky and creamy effect in sandalwood notes. To be combined with other sandalwood notes such as Sandela®, Bacdanol® or Polysantol®. Not to be put in candle base. Tends to flatten accords.
Year of discovery :
1976
Isomerism :
The isomer of Sandalore® formed during the synthesis has a ramified alcohol function, while Sandalore® has it on its main carbon chain. Sandalore® has two asymmetric carbons. It is therefore a mixture of isomers that is used even when Sandalore® is isolated. Adoxal® is a constitutional isomer of Sandalore®. Its smell is however radically different, as it is marine and aldehydic.
Synthesis precursor :
Sandalore® is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Sandalore® is not available in its natural state.
Synthesis route :
Sandalore® is synthesized by a condensation of Campholenal with Methyl Ethyl Ketone. Then, two compounds are obtained and catalytically hydrogenated to ensure a selectivity of this hydrogenation (the alcene function of the ring must not be hydrogenated). A mixture of two hydrogenated molecules is obtained. These two isomers can be separated by a fractional distillation, or used together, as their smell is similar. The term Sandalore® refers to only one of these two molecules.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
-
Restriction type : RESTRICTION
-
Cause of restriction : DERMAL SENSITIZATION
-
Amendment : 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,21 % 0,062 % 1,2 % 1,2 % 0,29 % 0,29 % 0,29 % 0,29 % 0,68 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 2,4 % 2,4 % 0,12 % 2,3 % 8,1 % 8,1 % 4,5 % 4,5 % No Restriction

