Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Peonile® - 30gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 10461-98-0
-
EINECS number : 423-740-1
-
FEMA number : Donnée indisponible.
-
Density : 1,03
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Base
-
Price Range : €€€
-
Appearance : Colorless liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 305°C
-
Detection Threshold : Donnée indisponible.
-
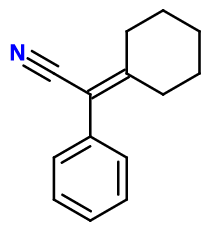
Molecular formula : C14H15N
-
Log P : 4
-
Molecular Weight : 197,28 g/mol
-
Fusion Point : 27°C
-
Flash Point : >93°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
The main characteristic of Peonile® is its tenacity. It ables to keep a rose note during a few weeks on a blopper.
Good synergy with Javanol®, in 80/20 proportions (rich, rosy and fruity sandalwood).
Stability :
Very stable in perfumes and in all functional bases, except very alkaline bases as liquid bleach.
Uses in perfumery :
Peonile® is used in fougere, hesperidic and floral compositions, to bring a geranium nuance. It brings tenacity to functional compositions, over all in fabric care bases.
Year of discovery :
1923
Isomerism :
Peonile® does not have any isomer used in pefumery.
Synthesis precursor :
Peonile® is not a precursor for the synthesis of another compound of olfactive interest.
Natural availability :
Peonile® is not found in nature. It is thus impossible to obtain it on a natural state.
Synthesis route :
Peonile® is obtained in one single step, using phenylacetonitrile and cyclohexanone, by forming an azeotrope (this means that they can mix on a vapour state, by heating the reaction medium). This reaction is done under alkaline conditions, and leads to the formation of water.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
This ingredient is not restricted for the 49th amendment

