Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Oxane - 30gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 59323-76-1
-
EINECS number : 261-699-8
-
FEMA number : 3578
-
Density : 1,05
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Head
-
Price Range : €€€€
-
Appearance : Colorless liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C8H16OS
-
Log P : Donnée indisponible.
-
Molecular Weight : 160,28 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 74°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Stability :
Stable in perfumes and diverse functional bases
Uses in perfumery :
Oxane is useful in fruity-exotic, blackcurrant, green and floral notes, as a top booster. Often used diluted.
Year of discovery :
1974
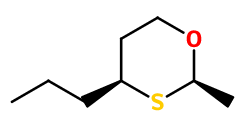
Isomerism :
Oxane is an enantiomerically pure molecule. Carbon 2 adopts an (R) conformation and carbon 4 conforms (S). These conformations are directly obtained during the synthesis of this compound. Other enantiomers are not used in perfumery.
Synthesis precursor :
Oxane is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Oxane is present in some fruits such as passion fruit, but Oxane used in perfumery is synthetic.
Synthesis route :
Oxane is synthesized by reaction between 3-mercaptohexanol and Acetaldehyde. This reaction causes a cyclization of the molecule, and therefore the sulfur compound Oxane.
Regulations & IFRA
This ingredient is not restricted

