Photo credits: ScenTree SAS
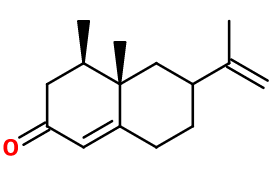
Nootkatone
(4R,4aS,6R)-4,4a-dimethyl-6-prop-1-en-2-yl-3,4,5,6,7,8-hexahydronaphthalen-2-one ; 1(10),11-eremophiladien-2-one ; 4,4alpha,5,6,7,8-hexahydro-6-isopropenyl-4,4alpha-dimethyl-2,3H-naphthalenone ; (4R,4aS,6R)-6-iso propenyl-4,4a-dimethyl-4,4a,5,6,7,8-hexahydro-3H-naphthalen-2-one

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Nootkatone - 30gr | - |
Visit website
|
- | - | - | - | - | - | |
|
|
Isobionics® Nootkatone 98 | - |
Visit website
|
Molecule | - | - | - | - | - |
General Presentation
-
CAS N° : 4674-50-4
-
EINECS number : 225-124-4
-
FEMA number : 3166
-
FLAVIS number : 07.089
-
JECFA number : 1398
-
Appearance : White solid
-
Density : 0,997
-
Volatility : Heart
-
Price Range : €€€€€
Physico-chemical properties
-
Molecular formula : C15H22O
-
Molecular Weight : 218,34 g/mol
-
Log P : 3,8
-
Fusion Point : 32°C
-
Boiling Point : 170°C (à 0,5 mmHg)
-
Detection Threshold : Entre 170 et 800 ppb (0,00008%) selon les personnes
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 110°C
Uses
Uses in perfumery :
Nootkatone can be used for fresh, citrusy or woody notes. Essential for a grapefruit note and to acidify rhubarb. Boosts top notes.
Year of discovery :
Data not available.
Natural availability :
Nootkatone can be isolated in its natural state from Grapefruit EO, which contains about 1 to 2%. Nevertheless, the Nootkatone used in perfumery is generally obtained synthetically.
Isomerism :
Nootkatone has three asymmetric carbons, one of which guides its enantiomer. Both enantiomers of the molecule have a similar smell. The racemic mixture is most often used, provided that the racemic mixture of Valencene is also used to synthesize it.
Synthesis precursor :
Nootkatone is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Nootkatone is synthesized by oxidation of a compound with a similar structure: Valencene, which can be isolated from Sweet Orange EO.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
-
Specified ingredients: notes
Nootkatone used as a fragrance ingredient should be at least 98% pure, with a melting point of at least 32°C. Lower purity grades may not be used as a fragrance ingredient.
Contributions from other sources
Nootkatone is found in natural extracts, but its natural contributions are not relevant for the fragrance ingredient specification mentioned above.


