Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
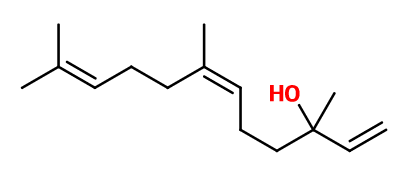
Nerolidol | 30034996 | Molecule | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 7212-44-4
-
EINECS number : 230-597-5
-
FEMA number : 2772
-
Density : 0,874
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Head
-
Price Range : €€
-
Appearance : Colorless liquid
-
FLAVIS number : 02.018
-
JECFA number : 1646
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 276°C
-
Detection Threshold : De l'ordre de 10 ppb à 10 ppm (0,0001%) selon les personnes
-
Molecular formula : C15H26O
-
Log P : 5
-
Molecular Weight : 222,37 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 125°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Comparing it with other rosy and green notes as Phenoxanol® and Rosacetol®, Nerolidol has a distinctive fruity and green note.
Stability :
Terpenes tend to polymerize by oxydation.
Uses in perfumery :
Nerolidol is used in all types of perfumery for orange, rose, honeysuckle and lily of the valley notes. Useful in citrus and woody-vanillic accords, to give an airy side and make the link between those two facets.
Year of discovery :
Discovered in 1923
Isomerism :
Nerolidol exists as two pairs of enantiomers, as it has an asymmetric carbon and a double bond that gives rise to two diastereoisomers. Synthetic Nerolidol is however a mixture of all these isomers. All have a similar smell, but they do not have the same physicochemical properties.
Synthesis precursor :
Nerolidol can be a precursor to the synthesis of other terpenes, for example by a Diels-Alder reaction. It is also a precursor for the synthesis of Farnesol, following an isomerization process.
Natural availability :
Dextrorotatory trans-Nerolidol is the major compound of cabreuva (84%), a shrub of Paraguay. This same dextrorotatory nerolidol is isolated from Dalbergia parviflora, an Asian liana.
Synthesis route :
Nerolidol is a sesquiterpene, often associated with Linalool, whose synthesis starts from this last compound. Linalool is converted to geranylacetone by reaction with Ethyl Acetoacetate, for example. A condensation of the obtained ketone with acetylene, followed by a Lindlar palladium catalysed hydrogenation allows to obtain Nerolidol.
Regulations & IFRA
This ingredient is not restricted

