Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Methyl Iso Eugenol | CL-802 | Natural | 100 | Eugenia caryophyllus | Clove Oil | Indonesia | more | 400 Kgs |
General Presentation
-
CAS N° : : 93-16-3
-
EINECS number : 202-224-6
-
FEMA number : 2476
-
Density : 1,05
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart
-
Price Range : €€
-
Appearance : Colorless to pale yellow liquid
-
FLAVIS number : 04.013
-
JECFA number : 1266
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 263°C
-
Detection Threshold : Donnée indisponible.
-
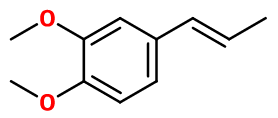
Molecular formula : C11H14O2
-
Log P : 3,05
-
Molecular Weight : 178,23 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 113°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
It can be used preferrentially to Methyl Eugenol, because Methyl Isoeugenol is not regulated.
Its smell is way more floral than the one of Methyl Eugenol.
Stability :
Become red under light and in alkaline bases. Thus, it is better not to use it in alkaline bases such as shower gels or shampoos.
Uses in perfumery :
Methyl Isoeugenol is used in tuberose and lily accord, to bring a characteristic note, in clove and in spicy flowers accords as carnation.
Year of discovery :
Data not available.
Isomerism :
Methyl Isoeugenol is a position isomer of Methyl Eugenol. Their smell is different : Methyl Isoeugenol is more floral, reminiscent of tuberose, while Methyl Eugenol is more spicy. Both are keeping an earthy and wet facet, more noticeable for Methyl Eugenol. Cis and trans isomers of Methyl Isoeugenol have a very close smell. This explains the general use of a racemic mix of these two isomers in perfumery.
Synthesis precursor :
Methyl Isoeugenol is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Methyl Isoeugenol can be found in small quantities in a few essential oils as Star Anise EO, Cinnamon Leaf EO, Mastic Absolute and some varieties of Damask Rose EO. It can be extracted from these oils and can be used on its natural state.
Synthesis route :
Methyl Isoeugenol is obtained in a synthetic way from Isoeugenol, thanks to a methylation reaction. This etherification reaction is done thanks to a Williamson synthesis, consisting in forming a sodium alcoholate, in the presence of pure sodium reacting with Isoeugenol. Then, the Isoeugenolate reacts with methyl chloride.
Regulations & IFRA
This ingredient is not restricted

