Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Anthranilate de Methyle - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 134-20-3
-
EINECS number : 205-132-4
-
FEMA number : 2682
-
Density : 1,166
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart
-
Price Range : €
-
Appearance : Colorless liquid to solid
-
FLAVIS number : 09.715
-
JECFA number : 1534
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 255°C
-
Detection Threshold : 0,12 ng/l air
-
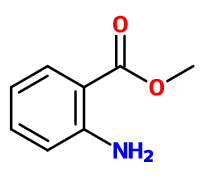
Molecular formula : C8H9NO2
-
Log P : 1,66
-
Molecular Weight : 151,16 g/mol
-
Fusion Point : 24°C
-
Flash Point : 123°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Methyl Anthranilate is more associated with Orange Blossom Absolute notes and fruity notes of wild strawberry than Dimethyl Anthranilate, closer to Mandarin Yellow EO.
Stability :
Methyl Anthranilate is well known for synthesizing Schiff bases by reacting espacially with aldehydes. These compounds can have an olfactive interest but are still a source of coloration in perfume concentrates and perfumes besides others. This is why this raw material is used is small quantities.
Exclusively stable in fabric conditioners, shampoos and hair conditioners
Uses in perfumery :
Methyl Anthranilate is used for wild strawberry and wild fruit notes, in orange blossom and exotic flower accords: gardenia, tuberose, jasmine.
Year of discovery :
1898
Isomerism :
Methyl Anthranilate does not have any isomer used in perfumery.
Synthesis precursor :
Methyl Anthranilate has the ability to easily react with aldehydes to form molecules called Schiff bases, which can serve as a perfumery ingredient or base for a Maillard reaction. For example, Aurantiol (Schiff Base) results from the reaction of this molecule with Hydroxycitronellal and is a raw material also used in perfumery. The Schiff base with Lilial® (Verdantiol) or the one with Triplal® (Vertosine) are also much used.
Natural availability :
Methyl Anthranilate is present in Grandiflorum Jasmine Absolute, Neroli EO, Ylang-Ylang Extra EO (and other ylang fractions), Champaca Absolute, in grapes and citrus fruits, from which it can be extracted in its natural state.
Synthesis route :
Methyl Anthranilate is synthesized either by esterification of anthranilic acid with methanol or by reaction between methanol and isatoic anhydride, releasing CO2.
Regulations & IFRA
This ingredient is not restricted

