Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Methyl Laitone - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 94201-19-1
-
EINECS number : 303-602-4
-
FEMA number : Donnée indisponible.
-
Density : 1,02
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Base
-
Price Range : €€€
-
Appearance : White solid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
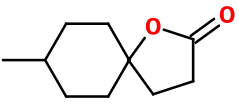
Molecular formula : C10H16O2
-
Log P : Donnée indisponible.
-
Molecular Weight : 168,24 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : >93°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
As it is very powerful, Methyl Laitone® is generally used in low quantity. It also is diluted in Dipropylene Glycol or Triethyl Citrate to get solubilized before usage.
Good synergy with Nectaryl®, in 50/50 propotions (peach cheesecake) ; with Dupical in 60/40 proportions (imaginary tonka flower) ; with Paradisamide® in 80/20 proportions (tropical fruits) ; with Kephalis® in 80/20 proportions (milky and minty incense).
Stability :
Stable in the major part of functional bases, except very acidic (detergent) and very alkaline bases (liquid bleach).
Uses in perfumery :
Methyl Laitone® brings a creamy, fruity and voluminous aspect to numerous accords. Used in white flower accords to bring a cosmetic effect, and in fruity peach or osmanthus notes. Brings a milky aspect to sandalwood accords, and gets on well with coumarin notes.
Year of discovery :
Data not available.
Isomerism :
Methyl Laitone® is a constitutional isomer of Jasmolactone®. These two molecules have lactone groups, giving a lactonic facet, but Methyl Laitone® is more reminiscent of coconut milk, as Jasmolactone® is of peach and jasmine.
Synthesis precursor :
Methyl Laitone® is not a precursor for the synthesis of another compound of olfactive interest.
Natural availability :
Methyl Laitone® is not found in nature. It is note possible to extract it from a natural product.
Synthesis route :
Synthesis of Methyl Laitone® can be carried out in one step by reacting acrylic acid with a large excess of para-methyl cyclohexanol (5 to 10 times more), in the presence of a catalytic amount of a free radical initiator as di-tertbutylperoxide, to a high temperature (145-155°C) to initiate the reaction.
Regulations & IFRA
This ingredient is not restricted

