Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Manzanate - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 39255-32-8
-
EINECS number : 254-384-1
-
FEMA number : 3488
-
Density : 0,86
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Head
-
Price Range : €€
-
Appearance : Colorless liquid
-
FLAVIS number : 09.526
-
JECFA number : 214
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 153°C
-
Detection Threshold : 0,003 ppb (0,0000000000003 %)
-
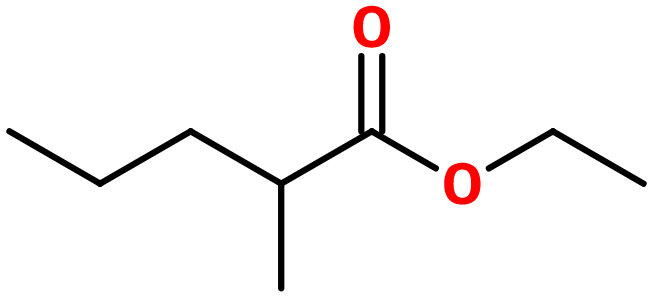
Molecular formula : C8H16O2
-
Log P : 2,65
-
Molecular Weight : 144,21 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 41°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Manzanate® is one of the commercial keys of modern perfumery. At the right dosage, it makes the perfume more addictive, wheither it is a functional or fine fragrance. Among these keys, Verdox®, Styrallyle acetate® and Ambroxan® can also be quoted.
Stability :
Only stable in Fabric conditioner, APC and shampoo bases. Particularly unstable in liquid fabric detergent and liquid bleach
Uses in perfumery :
Manzanate® is a key molecule in masculine perfumes. It is often used in small quantities to bring a strong fruity and addictive impact from the top note of the perfume, with a metallic and green note. Also used in apple, pineapple, exotic fruits and yellow fruits notes, to bring a heady and green effect.
Year of discovery :
1974
Isomerism :
Manzanate® has one asymmetric carbon in its strcture. (R)-2-methylpentanoate and (S)-2-methylpentanoate both exist. Manzanate® is a mixture of these two enantiomers. They are not used separately. On the other hand, Ethyl Caproate is a positional isomer of Manzanate®. Its smell is less elegant and closer to ripe pineapple, less green.
Synthesis precursor :
Manzanate® is not a precursor for the synthesis of another compound used in perfumes.
Natural availability :
Manzanate® is not reported as found in nature.
Synthesis route :
Manzanate® can be synthesized by an esterification reaction between 2-methylpentanoic acid and ethanol. This reaction can be catalyzed by the presence of a strong acid in a low quantity as concentrated sulfuric acid, to enhance the reaction yield. The use of chloro-2-methylpentanoic acid can also be a help.
Regulations & IFRA
This ingredient is not restricted

