Majantol®
2,2-dimethyl-3-(3-methylphenyl) propanol ; 2,2-dimethyl-3-(3-methylphenyl) propan-1-ol ; 3-(2,2-dimethyl-3-hydroxypropyl)toluol ; Lanjantol ; Lilivol ; Lily propanol ; Linlan alcohol ; Muguenol ; Trimethyl benzene propanol ; Trimethylbenzenepropanol
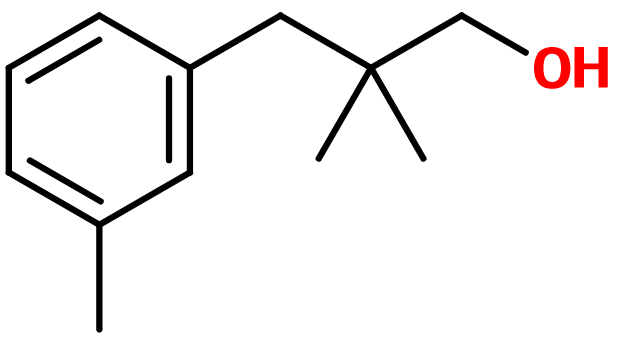
Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Majantol® - 30gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 103694-68-4
-
EINECS number : 403-140-4
-
FEMA number : Donnée indisponible.
-
Density : 0,97
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart
-
Price Range : €€
-
Appearance : Colorless liquid that solidifies at room temperature
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 289°C
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C12H18O
-
Log P : 3,38
-
Molecular Weight : 178,27 g/mol
-
Fusion Point : 24°C
-
Flash Point : 93°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Majantol® structure is close to Dimethyl Benzyl Carbinol. Its smell is less aldehydic and aqueous. Majantol® also has olfactive similarities with Lyal® and Hydroxycitronellal®, although it is more aqueous than theses molecules.
Stability :
Aromatic compounds are chromophorous. This means that they may color through time and in contact with alkaline bases.
Uses in perfumery :
Majantol® enters the floral-aldehydic molecules category, including Lilial® and Bourgeonal™ for example. It is used as it is slightly less regulated, for lily of the valley and other white flower notes, for a light effect, in association with other molecules as Hedione® or Florol®. It also brings a fresh nuance.
Year of discovery :
Patent N°3,531,585 (DE) published on Sept. 4, 1985 by Elektrochemisch Industrie GmbH
Isomerism :
Majantol® has a methyl group associated to carbon n°3 of its aromatic cycle, on a meta position. Molecules having this groupment on an ortho or para position are not used in perfumery, although they are isomers of Majantol® Phenoxanol® is a constitutional isomer of Majantol®, having a more rosy and green note.
Synthesis precursor :
Majantol® is not a precursor for the synthesis of another material used in perfumery.
Natural availability :
Majantol® is not reported as found in nature, and can thus not be extracted from any plant.
Synthesis route :
Majantol® can be prepared in two steps, starting with 3-methylbenzyl chloride, reacting it with 2-methylpropanal, in the presence of a catalysor as tetrabutylammonium iodide. An aldehyde is obtained and can be reducted, reacting with sodium tetrahydruroborate, to obtain the final product.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
-
Restriction type : RESTRICTION_SPECIFICATION
-
Cause of restriction : DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
-
Amendment : 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,034 % 0,2 % 0,025 % 1,7 % 0,43 % 0,061 % 0,039 % 0,013 % 0,0025 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,052 % 0,052 % 0,013 % 0,14 % 0,14 % 0,3 % 0,013 % 0,013 % 8,6 %
Comments :
2,2-Dimethyl-3-(3-tolyl)propan-1-ol should only be used as a fragrance ingredient if traces of organochlorine compounds are restricted. Total Chlorine, which can be measured by Atomic Absorption Spectroscopy, must not exceed 25 ppm in the raw material.

