Photo credits: ScenTree SAS
General Presentation
-
CAS N° : : 115-95-7
-
EINECS number : 204-116-4
-
FEMA number : 2636
-
Density : 0,901
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart
-
Price Range : €
-
Appearance : Colorless liquid
-
FLAVIS number : 09.013
-
JECFA number : 359
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 220°C
-
Detection Threshold : 1 ppm (0,0001%)
-
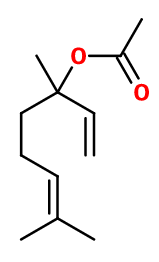
Molecular formula : C12H20O2
-
Log P : 3,93
-
Molecular Weight : 196,29 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 94°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
In comparision to Linalyl Propionate, Linalyl acetate is more representative of the smell of Bergamot EO on its agrestic and lavender aspects.
The detection threshold of Linalyl acetate is relatively high, compared with many other molecules used in perfumes.
Stability :
acetates may form acetic acid through time. Linalyl acetate is the most unstable acetate.
Uses in perfumery :
Linalyl acetate is used in bergamot and lavender accords. Brings a marked freshness in chypre (accompanied by patchouli, oakmoss and cistus among others) and oriental perfumes (accompanied by balsamic and vanilla notes).
Year of discovery :
Data not available.
Isomerism :
Linalyl acetate used in perfumery is a racemic mixture of its two (R) and (S) enantiomers. Both have a close smell. Geranyl acetate, Neryl acetate, Terpenyl acetate and Isobornyl acetate are isomers of Linalyl acetate. However, the first two are reminiscent of pear and rose, Isobornyl acetate is reminiscent of pine, and Terpenyl acetate also has a smell of Bergamot EO, although more fruity-sweet.
Synthesis precursor :
Linalyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Linalyl acetate is the major component of Bergamot EO, as it is present at about 30 to 40%. Therefore, it can be isolated to obtain natural Linalyl acetate. In addition, acetylation of Lavender EO and Petitgrain Bigarade EO enriches Linalyl acetate, as they contain a high level of Linalool. It is called hemi-synthetic Linalyl acetate. Linalyl acetate can also be removed by fractional distillation to produce it in its natural state.
Synthesis route :
Linalyl acetate is obtained synthetically by an esterification reaction between Linalool and acetic acid or acetic anhydride in an acid medium. Linalool is synthesized in two stages from Myrcene. Linalyl acetate can also be synthesized in two stages from Myrcene, by adding hydrochloric acid, catalysed by copper chloride II. The second step consists in reacting acetic acid with the intermediate product obtained in the presence of sodium acetate and dichloromethane.
Regulations & IFRA
This ingredient is not restricted



