Liffarome®
Methyl cis-3-hexenyl carbonate ; Methyl cis-3-hexenyl methanoate ; Greenarome ; Methyl cis-3-hexenyl formate ; Laniffarome ; Leafovert ; Muguet carbonate ; Vertelione ; Vertocarb ; Cis-3-hexenyl methyl carbonate ; Cis-3-hexenyl methyl methanoate ; Cis-3-hexenyl methyl formate
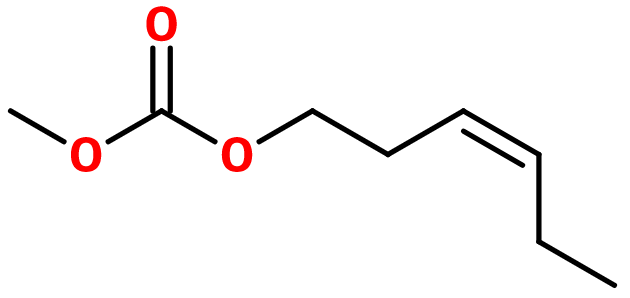
Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Liffarome - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 67633-96-9
-
EINECS number : 266-797-4
-
FEMA number : Donnée indisponible.
-
Density : 0,97
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Head/Heart
-
Price Range : €€€
-
Appearance : Colorless liquid
-
FLAVIS number : 09.838
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 193°C
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C8H14O3
-
Log P : 2,5
-
Molecular Weight : 158,2 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 77°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Liffarome® is one of the key green top and heart notes in all kinds of perfumes. It makes it possible to lengthen the green note for fruits and flowers.
Stability :
Esters may form their corresponding acid in stability
Uses in perfumery :
Liffarome® can be used in floral, fruity, violet leaf and mimosa notes, to bring natural and earthy effects from the head note of the perfume.
Year of discovery :
Data not available.
Isomerism :
Trans-3-Hexenyl methyl carbonate is a diastereoisomer of Liffarome®, but it is not used in perfumery.
Synthesis precursor :
Liffarome® is not a precursor for the synthesis of another compound used in perfumes.
Natural availability :
Liffarome® is not extracted from any plant as it is not found in nature.
Synthesis route :
Liffarome® synthesis can be carried out in two steps. The first one is an esterification reaction between phosgene and methanol, leading to methyl chlorocarbonate. Conditions for this reaction have to be careful because of the reactivity of phosgene, not to form dimethyl carbonate. Afterwards, a second esterification reaction can be carried out by reacting the intermediary product with Cis-3-Hexenol. No catalysis is needed for these reactions, because phosgene and methyl chlorocarbonate are both very reactive.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
This ingredient is not restricted for the 49th amendment

