Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Kephalis® - 30gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 36306-87-3
-
EINECS number : 252-961-2
-
FEMA number : Donnée indisponible.
-
Density : 0,947
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Head/Heart
-
Price Range : €€€
-
Appearance : Colorless liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 272°C
-
Detection Threshold : 3,9547 ng/l air
-
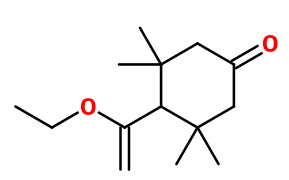
Molecular formula : C14H24O2
-
Log P : 4,3
-
Molecular Weight : 224,34 g/mol
-
Fusion Point : -80°C
-
Flash Point : 120°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
In comparision to other woody-ambergris notes also having a violet flower undernote, Iso E Super® is more reminiscent of Cedarwood Virginia EO than Vetiver Haiti EO, in the case of Kephalis®.
Stability :
Unstable in acidic products, except fabric conditioners, and in very alkaline detergents.
Uses in perfumery :
Kephalis® is used in woody, ambery, masculine fragrances, leather, suede, spicy and floral notes. Gives a leather and woody appearance from the head to the base.
Year of discovery :
Data not available.
Isomerism :
Kephalis® does not have any isomer used in perfumery.
Synthesis precursor :
Kephalis® is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Kephalis® is not available in its natural state.
Synthesis route :
Kephalis® is one of two compounds synthesized by a cyclodimerization reaction of mesityl oxide (or 4-methylpent-3-en-2-one), in the presence of boron trifluoride etherate, by reaction with ethyl orthoformate. The other compound formed during this reaction has no olfactory interest.
Regulations & IFRA
This ingredient is not restricted

