Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Javanol® - 30gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 198404-98-7
-
EINECS number : 427-900-1
-
FEMA number : 4776
-
Density : 0,947
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Base
-
Price Range : €€€€
-
Appearance : Colorless viscous liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : 2254
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 271°C
-
Detection Threshold : Donnée indisponible.
-
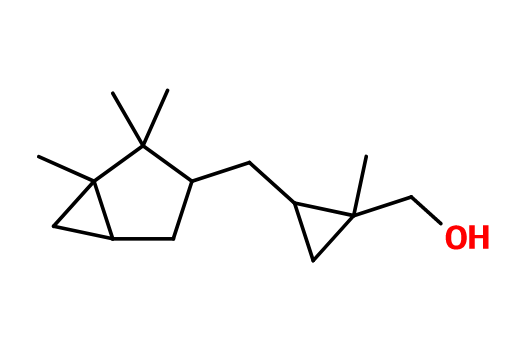
Molecular formula : C15H26O
-
Log P : 4,8
-
Molecular Weight : 222,37 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : >93°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
The detection limit of Javanol® is extremely low. This makes it one of the most used santal compounds, because it brings a milky and woody effect, even at a very small dosage.
Stability :
Unstable exclusively in acid cleaners and in bleach.
Uses in perfumery :
Javanol® is used for the same reasons as Bacdanol®, and for its aromatic facet, similar to thyme. Often used in small quantities as it is a very powerful and effective ingredient.
Year of discovery :
1996
Isomerism :
Javanol® has several asymmetric carbons that give rise to several possible isomers. Nevertheless, it is a mixture of isomers that is used in perfumery. In addition, Javanol® is a constitutional isomer of Polysantol®. These two compounds have a similar structure, and are used for similar reasons in perfumes. Javanol® still has a less milky note than Polysantol®, but is more powerful.
Synthesis precursor :
Javanol® is not involved in the synthesis of another compound of olfactory interest.
Natural availability :
Javanol® is not available in its natural state.
Synthesis route :
Javanol® is synthesized by a Simmons-Smith double reaction, consisting in cyclopropanation from 2-methyl-4-(2,2,3-trimethyl-3-cyclopenten-1-yl)-2-buten-1-ol. This reaction involves a catalysis with Zinc and Copper, as well as diodomethane, to convert a double bond into a cyclopropane group.
Regulations & IFRA
This ingredient is not restricted

