Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Jasmolactone® - 30gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 32764-98-0
-
EINECS number : 251-201-7
-
FEMA number : 4441
-
Density : 1,01
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Base
-
Price Range : €€€€
-
Appearance : Colorless liquid
-
FLAVIS number : 10.040
-
JECFA number : 1994
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 150°C (à 23 hPa)
-
Detection Threshold : Donnée indisponible.
-
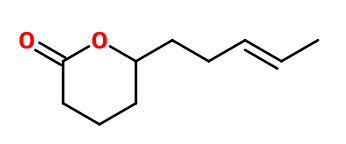
Molecular formula : C10H16O2
-
Log P : Donnée indisponible.
-
Molecular Weight : 168,24 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : >110°C (>230°F)
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Jasmolactone has a jasmine undernote that makes the difference with other lactones as Delta-Decalactone.
Stability :
Lactones tend to polymerize through time, making them more viscous and leading to a phase shift in alcohol.
Uses in perfumery :
Jasmolactone is used in luxury perfumery for reconstitutions of white flowers, for ambery notes and to give facets to a tea note.
Year of discovery :
1961
Isomerism :
Another molecule is also called δ-Jasmolactone, but is synthesized from 4-hydroxydec-7-enoic acid, which places the double bond of the final product on another carbon aside. The smell of this isomer is similar to the Jasmolactone described here. Gamma-Jasmolactone has a more fatty smell, close to peanut. Methyl Laitone® is a constitutional isomer of Jasmolactone, but has a coconut-like smell and a lactonic body.
Synthesis precursor :
Jasmolactone is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Jasmolactone is only present in trace amounts in Osmanthus Absolute, not allowing to obtain it in its natural state.
Synthesis route :
Like the other lactones, Jasmolactone, is synthesized by an intramolecular esterification reaction that involves 4-hydroxydec-8-enoic acid, in the presence of an acid catalyst. Then, the cyclization of this molecule gives rise to a δ-lactone.
Regulations & IFRA
This ingredient is not restricted

