Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Isoraldéine 70 - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 1335-46-2
-
EINECS number : 204-846-3
-
FEMA number : 2714
-
Density : 0,93
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart/Base
-
Price Range : €€
-
Appearance : Colorless to pale yellow liquid
-
FLAVIS number : 07.036
-
JECFA number : 404
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 238°C
-
Detection Threshold : 1,706 ng/l air
-
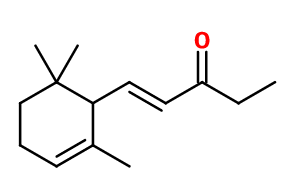
Molecular formula : C14H22O
-
Log P : 4,7
-
Molecular Weight : 206,3 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 124°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Isoraldeine 70® has a woodier note than Iralia®, less sweet than alpha-Ionone and less dusty than beta-Ionone.
Originally, the synthesis of some ionones gave birth to several organic waste, stored in barrels (fûts in French). The perfumer, Edmond Roudnitska, chose to use one of them in the creation of one of his biggest hits: fût n°5, which was subsequently regulated.
Then, Isoraldeine® served as a substitute for the note of fût n°5.
Methyl ionones are one of the 26 allergens in perfumery.
Stability :
Unstable in acid products, except fabric conditioners, and in very alkaline products.
Uses in perfumery :
Isoraldeine 70® is used in leather notes to bring a facet of violet flower, as it is more leather than Isoraldeine 95®. Can be used in a tea note, combined with Hédione®, Damascone-Beta® and Bergamot EO.
Year of discovery :
Data not available.
Isomerism :
As for conventional Ionones, the synthesis of alpha-Isomethylionone gives rise to other isomers of this molecule. beta-Isomethylionone has a more ambergris, orris and less fruity smell. Alpha-Irone and Cashmaeran are constitutional isomers of Isoraldeine®. Nevertheless, Cashmeran® has a distant smell, while Irone is closer to its violet aspect.
Synthesis precursor :
Isoraldeine 70® is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Isoraldeine 70® is not available in its natural state.
Synthesis route :
Isoraldeine 70® is composed of a predominantly methylionone isomer, called alpha-Isomethyl Ionone. The synthesis of methylionones is made from Citral and methyl ethyl ketone (instead of acetone for ionones). This synthesis step gives rise to two molecules called n-methyl pseudoionone and Isomethyl pseudoionone, both having cis and trans diastereoisomers. The ratio of one molecule relative to the other is favoured by the choice of the reaction catalyst: the stronger the catalyst base, the more Isomethyl pseudoionone will be favoured. For the cyclization step of Pseudoionone, as for Alpha-Ionone, the use of concentrated phosphoric acid favours alpha-Isomethylionone rather than the beta or gamma isomer. In general, each isomethyl ionone synthesis results in the formation of isomers of the desired molecule. In the case of Isoraldine 70®, its purity can reach 75% maximum (comparision with Isoraleine 95®).
Regulations & IFRA
This ingredient is not restricted

