Isopropyl myristate
IPM ; Propan-2-yl tetradecanoate ; Bisomel ; Crodamol IPM ; Deltyl extra ; Dermol IPM ; Edenor IEM 17 ; Emcol-IM ; Emerest 2314 ; Estergel ; Exceparl IPM ; Hallstar IPM ; Kessco IPM ; Kesscomir ; Liponate IPM ; 1-methylethyl tetradecanoate ; Isomyst ; Nikkol IPM-100 ; Promyr ; Propan-2-yl tetradecanoate ; Sinnoester MIP ; Starfol IPM ; Tegester ; Unimate IPM ; Isopropyl tetradecanoate ; 1-methylethyl tetradecanoate ; Wickenol 101
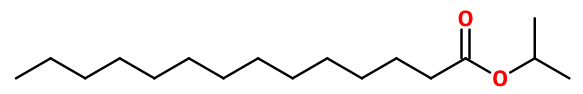
Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Myristate d'isopropyle - 500 mL | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 110-27-0
-
EINECS number : 203-751-4
-
FEMA number : 3556
-
Density : 0,85
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : NON TROUVE_N/A
-
Price Range : €
-
Appearance : Colorless liquid
-
FLAVIS number : 09.105
-
JECFA number : 311
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 193°C (à 20 mmHg)
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C17H34O2
-
Log P : 7,71
-
Molecular Weight : 270,45 g/mol
-
Fusion Point : 3°C
-
Flash Point : 152°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Stability :
Data not available.
Uses in perfumery :
Isopropyl Myristate is a solvent commonly found in perfume concentrates because it is used to dilute certain raw materials that are too viscous or pasty.
For stability reasons, Isopropyl Myristate is the most suitable candle and 100% natural candle base solvent. It can also be used in perfume concentrates for shower gels, shampoos, creams, body and hair oil and in dishwashing liquids.
Year of discovery :
Data not available.
Isomerism :
Isopropyl Myristate does not have any isomer used in perfumery.
Synthesis precursor :
Isopropyl Myristate does not interfere with the synthesis of another perfume compound.
Natural availability :
Isopropyl Myristate is found in some fruits, but is not extracted in its natural state for its use in perfumery.
Synthesis route :
Originally, Isopropyl Myristate is synthesized by an esterification reaction between myristic acid and isopropanol. This reaction requires a very large excess of isopropanol (15 times greater than the acid amount) and an acid catalysis in the presence of sulfuric acid, for example. Today, many synthetic routes are being developed to make this synthesis greener. These methods are always esterification reactions, but they use a cleaner biocatalysis, multiple reactors including enzymes and dehydrating substances, to shift the equilibrium of the reaction and obtain a purer MIP, while recycling the remaining reagents.
Regulations & IFRA
This ingredient is not restricted

