Iso E Super®
Amberonne® ; Orbitone® ; Arborone® ; 1-(2,3,8,8-tetramethyl-1,3,4,5,6,7-hexahydronaphthalen-2-yl)ethanone ; 1,2,3,4,5,6,7,8-octahydro-2,3,8,8-tetramethyl-2-acetonaphthone ; Amber fleur ; Isoamber super ; Amberfleur ; Ambergris ketone ; Amberix super ; Amberlan ; Ambroise super ; Anthamber ; Boisvelone ; Isocyclemone E ; Dimethyl myrcetone ; Hamber ; Iso gamma super ; Methyl cyclomyrcetone ; Patchouli ethanone ; Sylvamber ; Timbersilk ; Timbrone supra ; Iso velvetone
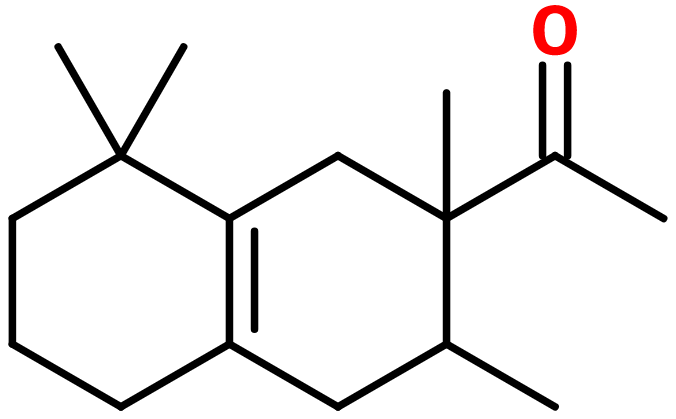
Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Iso E Super - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 54464-57-2
-
EINECS number : 259-174-3
-
FEMA number : Donnée indisponible.
-
Density : 0,964
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Base
-
Price Range : €€
-
Appearance : Colorless liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 290°C
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C16H26O
-
Log P : 5,65
-
Molecular Weight : 234,38 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 134°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
The name of Iso E Super® comes from the contraction of ISOcyclomyrcetone Ethanone, and the SUPERior quality which has been made accessible since the improvement of the manufacturing process. Often called 'bois-violette'.
From 1993, each company was free to produce its own Iso E Super®. The current production of the molecule is 1800 tons per year.
Stability :
Stable in perfumes and diverse functional bases
Uses in perfumery :
Iso E Super® is used in masculine, woody and ambery notes as a woody base. Allows to bring a light violet flower note to woody accords.
Year of discovery :
Discovered in 1956. Iso E Super® was first patented in 1973, by scientists John B. Hall and James M. Sanders.
Isomerism :
Iso E Super® is composed by more than 20 isomeric molecules. The molecule called Isocyclemone in its pure state, has almost no smell, but the Iso E Super® called '' Plus '', so-called Arborone, found at between 2 and 5% in the commercialised Iso E Super®, has a very strong smell (10 000 times more powerful than Iso E Super), and is responsible for the smell of Iso E Super®. Iso E Super® Plus alone is not marketed because its production is too expensive.
Synthesis precursor :
Iso E Super® is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Iso E Super® is not available in its natural state.
Synthesis route :
Iso E Super® is synthesized by a Diels-Alder reaction between Myrcene and 3-methyl-3-penten-2-one in the presence of aluminum chloride. The cyclization of the intermediate product obtained is made thanks to the action of phosphoric acid. This cyclization gives birth to two isomers : Iso E Super® and Arborone, in 95/5 proportions.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
-
Restriction type : RESTRICTION
-
Cause of restriction : DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
-
Amendment : 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,41 % 1,1 % 0,41 % 20 % 5,1 % 0,56 % 0,76 % 0,19 % 0,0093 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,67 % 0,67 % 0,19 % 2,4 % 2,4 % 6,6 % 0,19 % 0,19 % No Restriction

