Hydroxycitronellal
Laurinal® ; Cyclosia® ; Cyclosia Base® ; 7-hydroxy-3,7-dimethyloctanal ; Citronellal hydrate ; Citronellaldehyde ; Oxydihydrocitronellal ; Hidroxycitronellal ; 7-hydroxy-3,7-dimethyloctanal ; Lily aldehyde ; Muguet synthetic ; Muguettine principle ; Oxdihydrocitronellal
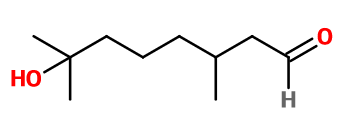
Photo credits: ScenTree SAS
General Presentation
-
CAS N° : : 107-75-5
-
EINECS number : 203-518-7
-
FEMA number : 2583
-
Density : 0,942
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart
-
Price Range : €€
-
Appearance : Colorless liquid
-
FLAVIS number : 05.012
-
JECFA number : 611
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 257°C
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C10H20O2
-
Log P : 1,42
-
Molecular Weight : 172,27 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 113°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Hydroxycitronellal is one of the 26 allergens in perfumery. It is also regulated by the IFRA. Therefore, it is often replaced by Florol®, which is not regulated.
Used for the first time in 1912, in Quelques fleurs by Houbigant.
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell.
Terpenes tend to polymerize by oxydation.
Liquefies shower gel bases.
Uses in perfumery :
Hydroxycitronellal is used in white flowers notes: lily of the valley, lilac. Tends to be more and more restricted.
Year of discovery :
Discovered in 1905.
Isomerism :
Hydroxycitronellal has an asymmetric carbon that gives rise to two possible enantiomers with a similar smell. Florol® is a constitutional isomer of Hydroxycitronellal. Both are used in particular in lily of the valley reconstructions. However, Florol® tends to replace Hydroxycitronellal in formulas, as it is not regulated.
Synthesis precursor :
Hydroxycitronellal is a precursor molecule of the synthesis of Aurantiol (Schiff Base), by reaction with Methyl Anthranilate .
Natural availability :
Hydroxycitronellal is not available in its natural state.
Synthesis route :
Hydroxycitronellal can be synthesized from several different reagents. The final product can be obtained from Citronellal in an acid medium and by reaction with sodium carbonate. From Citronellol, an acid hydrolysis reaction in the presence of sulfuric acid allows to obtain a diol, capable of undergoing a dehydrogenation catalysed by copper and zinc, for example. This method offers a high purity and a very good yield. From Myrcene, a reaction with a dialkylamine allows to synthesize 7-hydroxygeranyl dialkylamine, after an acid hydrolysis. After that, the presence of palladium (II) phosphine in a catalytic amount allows to change the place of the double bond in order to obtain a compound called enamine. A last acid hydrolysis of this compound allows to obtain Hydroxycitronellal.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
-
Restriction type : RESTRICTION
-
Cause of restriction : DERMAL SENSITIZATION
-
Amendment : 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,38 % 0,11 % 2,3 % 2,1 % 0,53 % 0,53 % 0,53 % 0,53 % 1,2 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 4,3 % 4,3 % 0,22 % 4,1 % 15 % 15 % 8,2 % 8,2 % No Restriction
Comments :
This ingredient is part of the Schiff base (Hydroxycitronellal-Indole (or Indolene 50%) - N°CAS : 68527-79-7) and induces the application of IFRA regulations for 63,5% of the Schiff base usage. This ingredient is part of the Schiff base (Hydroxycitronellal methyl anthranilate (or Aurantiol, Aurantium, Aurantoin) - N°CAS : 89-43-0) and induces the application of IFRA regulations for 56,4% of the Schiff base usage. Please also refer to the IFRA Annex II for more information

