Helional®
Aquanal® ; Heliobouquet® ; Heliogan® ; 3-(1,3-benzodioxol-5-yl)-2-methylpropanal ; Cetonial ; Floramelon ; Florial ; Neohelial ; Heliofresh ; Heliolan ; Helionix ; Heliopropanal ; Alpha-methyl-1,3-benzodioxole-5-propanal ; Alpha-methyl-1,3-benzodioxole-5-propionaldehyde ; 2-methyl-3-(3,4-methyenedioxyphenyl)propionaldehyde ; Alpha-methyl-3,4-(methylene dioxy) hydrocinnamaldehyde ; 3-(3,4-methylenedioxyphenyl)-2-methylpropanal ; Neohelial ; Ocean propanal ; Tropional
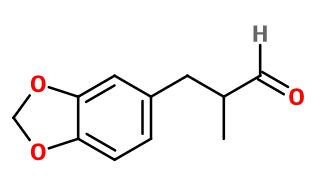
Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Helional - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 1205-17-0
-
EINECS number : 214-881-6
-
FEMA number : 4599
-
Density : 1,163
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart/Base
-
Price Range : €€
-
Appearance : Colorless liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : 2212
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 282°C
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C11H12O3
-
Log P : 1
-
Molecular Weight : 192,21 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 100°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
The smell of Helional® is less aldehydic than Cyclamen Aldehyde, less fruity than Melonal® and less marine than Calone®. Helional® is also less powerful than these three compounds.
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell.
Can react with Methyl Anthranilate to form a Schiff base that colors through time.
Uses in perfumery :
Helional® is used in marine and juicy fruits accords, or for a light and ozonic note. Gives volume to a perfume or accord. Often used in functional fragrance.
Year of discovery :
Discovered in 1958. Patent N°3,008,968 published in february,11 1958 by Muus G.J. Beets and Harm van Essen for IFF
Isomerism :
Helional® has an asymmetric carbon. The racemic mixture of the two enantiomers of the molecule is most often used. The (R) isomer is responsible for a fairly sweet aldehyde lily of the valley smell, while the (S) isomer is more ozonic, green and fruity.
Synthesis precursor :
May form a Schiff base with Methyl Anthranilate, called ''Corps Oranger ''.
Natural availability :
Helional® is not available in its natural state.
Synthesis route :
Helional® can be synthesized from Heliotropin by a condensation reaction with propanal, followed by a catalytic hydrogenation of the double bond that has formed.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
-
Restriction type : RESTRICTION
-
Cause of restriction : DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
-
Amendment : 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,12 % 0,25 % 0,039 % 2,6 % 0,39 % 0,077 % 0,077 % 0,026 % 0,62 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,077 % 0,077 % 0,026 % 0,15 % 0,15 % 0,62 % 0,026 % 0,026 % 12 %
Comments :
This ingredient is part of the Schiff base (Helional-methyl anthranilate (or Helioforte) - N°CAS :111753-60-7) and induces the application of IFRA regulations for 59,1% of the Schiff base usage. Please also refer to the IFRA Annex II for more information

