Hedione®
Methyl Dihydrojasmonate ; Cepionate® ; MDJ Super® ; Kharismal® ; Methyl 3-oxo-2-pentylcyclopentane acetate ; Claigeon ; Jasmodione ; MDJ ; Methyl (2-pentyl-3-oxocyclopentyl) acetate ; Methyl 2-pentyl-3-oxo-1-cyclopentyl acetate ; Methyl (3-oxo-2-pentylcyclopentyl) acetate ; Supercepionate ; Paradisone®
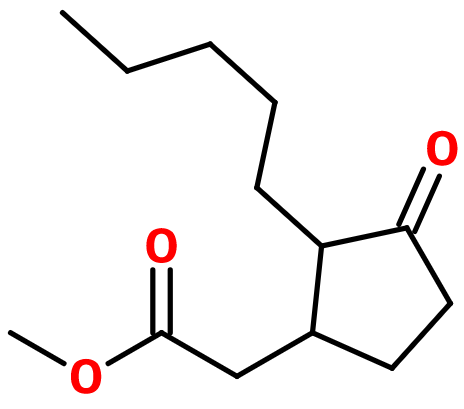
Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Hedione - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 24851-98-7
-
EINECS number : 246-495-9
-
FEMA number : 3408
-
Density : 1,005
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart
-
Price Range : €€
-
Appearance : Colorless liquid
-
FLAVIS number : 09.520
-
JECFA number : 1898
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 302°C
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C13H22O3
-
Log P : 2,93
-
Molecular Weight : 226,31 g/mol
-
Fusion Point : <20°C
-
Flash Point : 148°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Stability :
Stable in perfumes and diverse functional bases
Hedione® HC is quite unstable and tends to convert into trans-Hedione®.
Uses in perfumery :
Hedione® is used in jasmine reconstitutions, and all kinds of accords, to give volume to the whole, to accompany and give tenacity to the perfume. Allows, in association with Bergamot EO, Beta-Ionone and Damascone-beta, to recreate a tea note for example. Present in a large majority of commercial fragrances.
Year of discovery :
Discovered in 1960, Hedione® was sold at that time for 7500 suiss francs per kilogram. This price really decreased. Patent N°CH382731A published on feb 25, 1960 by Demole.E et Lederer.E for Firmenich et Cie. ''Hedione® '' tradename has been published and protected by Firmenich SA since 04/09/1972 (brand N°392334)
Isomerism :
Hedione®, commonly used in perfumery, is actually a 9:1 mixture of trans:cis diastereoisomers of the molecule. However, cis-Hedione® is the most powerful isomer. Its detection threshold is seventy times lower than trans-Hedione®. Thus, some Hedione® enriched in cis isomer are marketed: Cepionate® (30% cis), Kharismal® (60% cis) and Hedione® HC (for ''high cis '') (75% cis). Nevertheless, this Hedione® tends to be more unstable than the classic Hedione®, as it isomerizes in its trans form, more stable. Hedione® HC is prepared by a specific distillation technique that involves sodium carbonate. Another method is to hydrogenate the corresponding cyclopenteneacetic acid by a ruthenium II catalysis, followed by a methanol esterification of the acid function.
Synthesis precursor :
The classic Hedione® is a mixture of isomers, it is used to isolate Hedione® HC (High Cis), a superior olfactory quality and power (also to be notes : Cepionate®, Kharismal® and Paradisone®)
Natural availability :
Hedione® is present in trace amounts in Grandiflorum Jasmine Absolute and in some teas. It is nevertheless exclusively synthetic Hedione® that is used in perfumery.
Synthesis route :
Hedione® is synthesized from a condensation between cyclopentanone and valeraldehyde, followed by an isomerization of the intermediate product obtained to give 2-pentyl-2-cyclopenten-1-one. This molecule can undergo a Michael addition by reacting it with an alkyl malonate. A hydrolysis followed by a decarboxylation and finally an esterification (using methanol and an acid catalysis) of the acid function that has formed, allows to synthesize the final product, Hedione®.
Regulations & IFRA
This ingredient is not restricted

