Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Habanolide® - 30gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 111879-80-2
-
EINECS number : 422-320-3
-
FEMA number : Donnée indisponible.
-
Density : 0,964
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Base
-
Price Range : €€€
-
Appearance : Colorless liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 307°C
-
Detection Threshold : 0,5 ng/l air
-
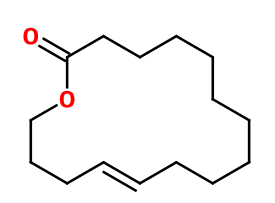
Molecular formula : C15H26O2
-
Log P : 6,2
-
Molecular Weight : 238,37 g/mol
-
Fusion Point : -33°C
-
Flash Point : >100°C (>212°F)
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
In comparision to other musks, Habanolide® has an orange blossom note making the difference with other ones. This facet is only percievable when the molecule macerates a few weeks in its base.
Stability :
Musks are very stable, as in alcoholic and in functional fragrances
Uses in perfumery :
Habanolide® is used in white floral notes, orange blossom and woody notes. Useful in marine and amber notes.
Used only in fine fragrance, especially for colognes.
Year of discovery :
Discovered in 1969. ''Habanolide® '' tradename has been published and protected by Firmenich SA since 05/03/1992 (brand N°584055)
Isomerism :
Two diastereoisomers of Habanolide® exist. The molecule called Habanolide® is the (E) (trans) diastereoisomer of the molecule. The diastereoisomer (Z) is less used, but has a similar smell. The mixture of the two isomers is also widely used.
Synthesis precursor :
Habanolide® is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Habanolide® is not available in its natural state.
Synthesis route :
Habanolide® is a macrocyclic musk, close to Exaltolide®. Therefore, it can be synthesized in a similar way, by enlarging the Cyclododecenone cycle.
Regulations & IFRA
This ingredient is not restricted

