Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Geraniol - 30 Gr | - | - | - | - | - | - | more | - | |
|
|
Geraniol Natural 85%+ | CT-201 | Natural | 85 | Cymbopogon winterianus jowitt | Citronella Oil | Indonesia | more | 50 Kgs | |
|
|
Geraniol Ex Palamarosa | 4410000101 | Naturel | - | - | - | - | more | - | |
|
|
GERANIOL | F1885 | Extrait | - | Cymbopogon martinii (Roxb.) Will. Watson var. motia | Pas d'informations disponibles. | France | more | - | |
|
|
Geraniol 60 | 30035070 | Molecule | - | - | - | - | more | - | |
|
|
Geraniol 60 BMBCert™ | 30770690 | Molecule | - | - | - | - | more | - | |
|
|
Geraniol Extra | 30035071 | Molecule | - | - | - | - | more | - | |
|
|
Geraniol Extra BMBCert™ | 30770691 | Molecule | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 106-24-1
-
EINECS number : 203-377-1
-
FEMA number : 2507
-
Density : 0,878
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Head/Heart
-
Price Range : €
-
Appearance : Colorless liquid
-
FLAVIS number : 02.012
-
JECFA number : 1223
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 229°C
-
Detection Threshold : 4 à 75 ppb (0,0000075%)
-
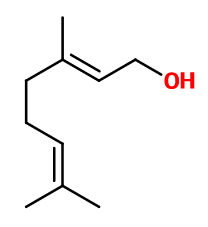
Molecular formula : C10H18O
-
Log P : 2,5
-
Molecular Weight : 154,25 g/mol
-
Fusion Point : -15°C
-
Flash Point : 113°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Geraniol is one of the 26 allergens in perfumery, unlike Nerol, which is not considered as an allergenic.
The smell of Geraniol is more citric and less metallic than the one of Nerol.
Stability :
Terpenes tend to polymerize by oxydation.
Uses in perfumery :
Geraniol is used in rose and geranium accords, for its fresh and rosy facet. Used to give more facets to fruity notes. Gives freshness to head notes and a rosy heart.
Year of discovery :
1926
Isomerism :
Geraniol is a diastereoisomer of Nerol. However, the smell of Nerol is closer to Neroli EO and Magnolia Flower EO than Geraniol, which is more like Dmask Rose EO. Several other constitutional isomers of Geraniol exist: Linalool, Eucalyptol and Rose Oxide are among them. All have very different smells.
Synthesis precursor :
Geraniol is a precursor to the synthesis of many compounds of olfactory interest. A rearrangement in the presence of a copper catalyst allows to obtain Citronellal. The presence of a mineral acid allows to cyclize the molecule, to obtain Cyclogeraniol for example, if the alcohol function is protected. A partial hydrogenation gives Citronellol and a total hydrogenation synthesizes Tetrahydrogeraniol. Citral can be obtained by oxidation of the molecule. Finally, several esters can be synthesized by reaction with various carboxylic acids.
Natural availability :
Natural Geraniol is mainly obtained from Palmarosa EO (contains up to 85% Geraniol), all kinds of Geranium EO and from Lemongrass EO (about 60% yield for this oil).
Synthesis route :
Geraniol can be obtained in different ways. A first solution is a hydrogenation of Citral, to synthesize a mixture of Geraniol and Nerol, often intermediate to the synthesis of vitamin A. Moreover, a pyrolysis of beta-Pinene allows to obtain Myrcene, which is converted into geranyl, neryl and linalyl chloride by adding hydrochloric acid (catalysed by copper chloride I and a quaternary ammonium salt, for example). The reaction of these intermediates with sodium acetate, followed by a saponification of the esters obtained, enables to obtain pure synthetic Geraniol, after a fractional distillation. Finally, Linalool can be subjected to a catalysis containing orthovanadates, to obtain Geraniol and Nerol, separable by fractional distillation. The majority of synthetic Geraniol produced today remain synthesized from alpha-Pinene, which gives Linalool, converted to linalyl borates and rearranged into geranyl and neryl borates, in the presence of a vanadate catalysis. Geraniol is obtained by hydrolysis of the esters and then by a fractional distillation.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
-
Restriction type : RESTRICTION
-
Cause of restriction : DERMAL SENSITIZATION
-
Amendment : 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,85 % 0,25 % 5,1 % 4,7 % 1,2 % 1,2 % 1,2 % 1,2 % 2,8 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 9,6 % 9,6 % 0,5 % 9,2 % 33 % 33 % 18 % 18 % No Restriction

