Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Galaxolide DPG - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 1222-05-5
-
EINECS number : 214-946-9
-
FEMA number : Donnée indisponible.
-
Density : 1,037
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Base
-
Price Range : €
-
Appearance : Colorless viscous liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 304°C
-
Detection Threshold : 0,9 ng/l air
-
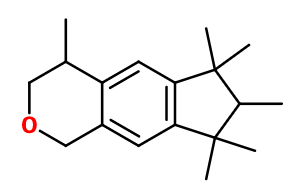
Molecular formula : C18H26O
-
Log P : 6
-
Molecular Weight : 258,41 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : >100°C (>212°F)
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Often, Galaxolide® is diluted in solvent as it is very viscous : Benzyl Benzoate, Isopropyl Myristate, Triethyl Citrate, Dipropylene Glycol.
Like some other polycyclic musk, Galaxolide® is not biodegradable and causes major pollution problems. As a result, some companies have decided to ban this compound from their products.
Stability :
Musks are very stable, as in alcoholic and in functional fragrances
Uses in perfumery :
Galaxolide® gives hold and trail to a perfume. It is one of the freshest and most heady musks. To be combined with other musks to multiply the facets. Widely used in detergents and fabric softeners. Preferential use in laundry. Often used as a replacement for all other musks in the case of a reformulation at a lower cost.
Year of discovery :
Discovered in 1962.
Isomerism :
Galaxolide® contains several asymmetric carbons that give rise to several enantiomers. Thus, Galaxolide® used in perfumery is actually a mixture of at least seven isomeric molecules with different volatilities. Galaxolide® and Tonalide® are constitutional isomers and have a fairly similar overall structure. Both have a relatively similar smell, as they belong to the same family of musks.
Synthesis precursor :
Galaxolide® is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Galaxolide® is not available in its natural state.
Synthesis route :
Galaxolide® can be synthesized from tert-amylene by reaction with alpha-methylstyrene, to obtain 1,1,2,3,3-pentamethylindane. This intermediate molecule can be hydroxyalkylated by reaction with the propylene oxide, during a Friedel and Craft reaction, catalysed by aluminum chloride, for example. The alcohol function of the second intermediate product obtained during the previous process can be cyclized by reaction with formaldehyde, either in an alcoholic medium or with an acid anhydride, to obtain the final compound, Galaxolide®.
Regulations & IFRA
This ingredient is not restricted

