Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Ethyl Acetoacetate - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 141-97-9
-
EINECS number : 205-516-1
-
FEMA number : 2415
-
Density : 1,03
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Head
-
Price Range : €
-
Appearance : Colorless liquid
-
FLAVIS number : 09.402
-
JECFA number : 595
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 183°C
-
Detection Threshold : 520 ppb (0,000052%)
-
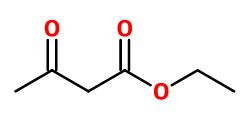
Molecular formula : C6H10O3
-
Log P : -0,2
-
Molecular Weight : 130,14 g/mol
-
Fusion Point : -43°C
-
Flash Point : 64°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Stability :
May form acetoacetic acid through time, under the effect of heat. It also opacifies shower gel and shampoo bases.
Uses in perfumery :
Ethyl Aceto acetate allows to give power and volatility to floral notes. Very useful also in fruity notes of kiwi or strawberry for example. Can give an artificial facet if overdosed.
Year of discovery :
Data not available.
Isomerism :
Ethyl Aceto acetate does not have any isomer used in perfumery.
Synthesis precursor :
Ethyl Aceto acetate is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Ethyl Aceto acetate can be found and extracted naturally from Coffee Absolute in very small quantities.
Synthesis route :
Ethyl Aceto acetate can be synthesized by an esterification reaction between Acetoacetic Acid and ethanol. Acetoacetic Acid is obtained before this process by an aldolization reaction of formic acid on the enolic form of acetone (obtained by acid catalysis).
Regulations & IFRA
This ingredient is not restricted

