Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Ebanol® - 30gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 67801-20-1
-
EINECS number : 267-140-4
-
FEMA number : 4775
-
Density : 0,9
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Base
-
Price Range : €€
-
Appearance : Colorless liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : 2220
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 283°C
-
Detection Threshold : Donnée indisponible.
-
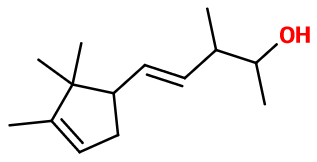
Molecular formula : C14H24O
-
Log P : 4,9
-
Molecular Weight : 208,34 g/mol
-
Fusion Point : <-50°C
-
Flash Point : 108°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Chemical structure of Ebanol® can remind the one of Sandalore®. Sandalore® simply does not have the carbon-carbon double bond found in Ebanol®, and which leads Ebanol® to be a mixture of isomers. Sandalwood molecules have most of the time this kind of molecular body : Bacdanol®, Polysantol® and Javanol® also have this sandalwood note.
Stability :
Stable in perfumes and in various bases, except stong acidic (detergents, antiperspirants) and very alkaline (detergents, liquid bleach) bases.
Uses in perfumery :
Ebanol® is used to bring volume and elegance to woody accords, with a typical sandalwood and diffusive note. It brings great tenacity in perfumes.
Year of discovery :
1986
Isomerism :
Ebanol® is a mixture of four diastereoisomers. These isomers appear during the synthesis of this material and are due to three asymmetric carbons and one double bond that is giving birth to two isomers inside the molecule. Among these isomers, trans-(E)-Ebanol® and cis-(Z)-Ebanol® are important. Then, a mixture of these isomers is used in perfumery.
Synthesis precursor :
Ebanol® is not used for the synthesis of any other material used in perfumery.
Natural availability :
Ebanol® is not found in nature. Thus, it cannot be extracted from any plant.
Synthesis route :
Ebanol® can be synthesized in three steps. The first one is a condensation of Campholenaldehyde with 2-butanone. The obtained intermediary product is isomerized by adding potassium tert-butylate, diluted in dimethyl formamide. The last step is a reduction of the product with sodium tetrahydruroborate, leading to four stereoisomers (see ''Isomerism '' paragraph).
Regulations & IFRA
This ingredient is not restricted

